Carbon dioxide and cardiac output as major contributors to cerebral oxygenation during apneic oxygenation
Myron Yaster MD and Ken Brady MD
I vividly remember the first time I saw the use of apneic oxygenation and simply couldn’t believe it. How in the world did this technique work and how could oxygen saturation remain above 90% without ventilation for as long as an hour? Forty years later, I still don’t think the physiology is completely understood. Nevertheless, this technique which was initially a novelty, has, over the past decade, dramatically increased in both the ORs and ICUs. Indeed, it is now recommended for all difficult airway intubations (and extubations) including routine intubations in all newborns, and in a wide field of interventions and operations, where a tracheal tube would be hindering to the surgeon or interventionalist.
In today’s PAAD Kaiser et al.1 in a secondary analysis of a randomized controlled trial2, sought to “to provide further evidence for the safety of apnoeic oxygenation and to increase the understanding of the association between cerebral oxygenation and cardiac output, carbon dioxide, age, gender and haemoglobin.”1 Because the authors used Near-infrared spectroscopy (NIRS) and extensively studied cerebral oxygen oxygenation, cardiac output, arterial partial pressure of carbon dioxide (PaCO2) and oxygen (PaO2) during apneic oxygenation, I asked my good friend and colleague, Dr. Ken Brady, who is amongst the foremost experts in the field of cerebral blood flow and autoregulation to assist. Myron Yaster MD
Original article
Kaiser HA, Bauer T, Riva T, Greif R, Riedel T, Theiler L, Nabecker S. Carbon dioxide and cardiac output as major contributors to cerebral oxygenation during apnoeic oxygenation. Sci Rep. 2024 Feb 13;14(1):3617. doi: 10.1038/s41598-023-49238-3. PMID: 38351038; PMCID: PMC10864331.
Original article
Riva T, Greif R, Kaiser H, Riedel T, Huber M, Theiler L, Nabecker S. Carbon Dioxide Changes during High-flow Nasal Oxygenation in Apneic Patients: A Single-center Randomized Controlled Noninferiority Trial. Anesthesiology. 2022 Jan 1;136(1):82-92. doi: 10.1097/ALN.0000000000004025. PMID: 34758057.
“Between March 2018 and December 2019 this single-centre study included adult patients, ASA physical health status I to III, who were planned to undergo general anaesthesia for elective surgery at the Department of Anaesthesiology and Pain Medicine, Bern University Hospital, Inselspital, Bern, Switzerland. Patients who were considered vulnerable towards hypercapnia and hypoxia due to their comorbidities (e.g., known coronary heart disease, peripheral occlusive arterial disease, anaemia, pregnancy, pulmonary hypertension, increased intracranial pressure, obstructive sleep apnoea, nasal obstruction or hyperkalaemia) were excluded from the study. The experiment was terminated once any of the following criteria were met: Arterial oxygen saturation (SpO2)<92%, transcutaneous pCO2 (tcpCO2)>100 mmHg, pH<7.1, potassium (K+)>6.0 mmol/L, or apnoea time reaching 15 minutes. Patients were monitored with standardised anaesthesia monitoring, consisting of ECG, pulse oximetry, invasive blood pressure monitoring via peripheral arterial line, end-tidal O2 and end-tidal CO2 measurements, transcutaneous pCO2 measurement (TCM, Radiometer, Talwil, Switzerland), train of four (TOF), electrical impedance tomography (PulmoVista 500; Draeger, Luebeck, Germany) and EEG surveillance (Narcotrend®, Hannover, Germany). To measure cerebral oxygenation we used the forehead near-infrared spectroscopy (NIRS) technique of the NIRO monitor (Niro-200NX, Hamamatsu, Tokyo, Japan), which delivers the tissue oxygenation index (TOI). The TOI was continuously recorded every second. Cardiac output was obtained using pulse contour analysis of the arterial waveform by a LiDCO device (LiDCO, London, UK) with measurements every second. The LiDCO was not calibrated with a Lithium bolus, as the trend of cardiac output was considered important and not the absolute values. Throughout the study, serial arterial blood samples for blood gas analysis (ABL 800, Radiometer, Krefeld, Germany) were drawn and analysed in our central laboratory: awake, immediately afer apnoea start and one minute later, then every two minutes during the 15 min apnoeic period.”1
“Patients were assigned to one of 5 groups: 1) Minimal-fow group: 0.25 L/min oxygen via endotracheal tube; 2) Low-fow group: 2 L/min oxygen + continuous jaw thrust; 3) Medium-fow group: 10 L/min oxygen + continuous jaw thrust; 4) High-fow group: 70 L/min oxygen + continuous jaw thrust; 5) Control group: 70 L/min oxygen + continuous laryngoscopy with a McGrath MAC video laryngoscope.”1
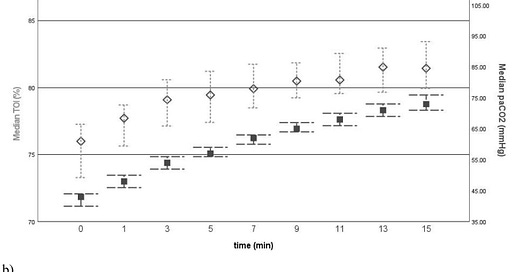
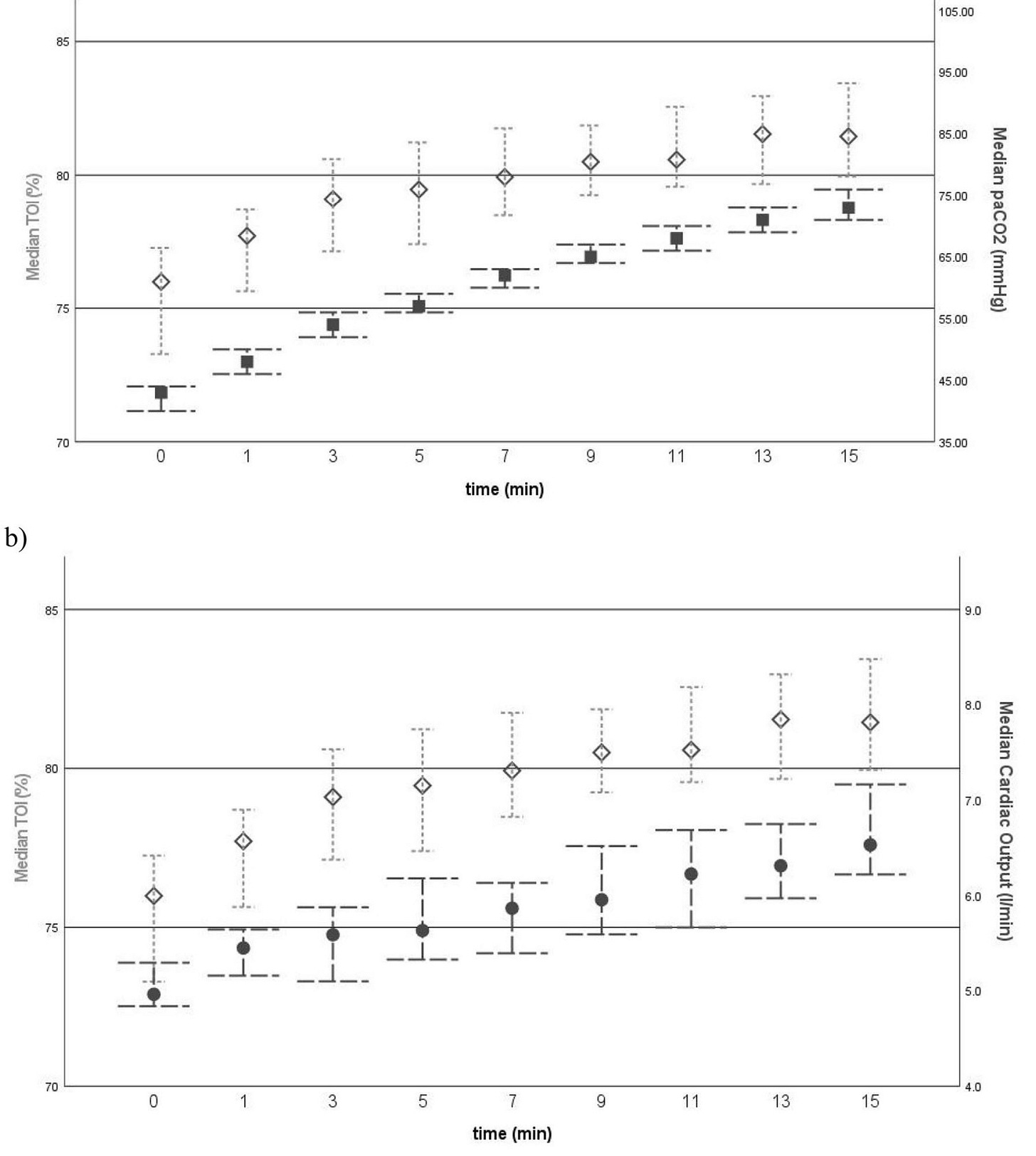
What did they find? “We observed a steady and significant increase in cerebral oxygenation of 0.5%/min during 15 min of apnoeic oxygenation.”1 The increase in cerebral oxygenation was primarily caused by the increasing PaCO2 and cardiac output through the study period (figure below). On the other hand, previous studies have demonstrated that cerebral blood flow is primarily regulated by arterial blood pressure and not cardiac output.3
Figure legend: “Comparative graphs of (a) medians of tissue oxygenation index (TOI) (shown as diamonds) versus arterial partial pressure of carbon dioxide (paCO2) (shown as squares) over time, as well as (b) medians of TOI verus cardiac output (CO) (shown as dots) over time; 95% confdence intervals are presented as bars. Thus, apneic oxygenation is associated with rising PaCO2 levels which significantly increased cardiac output and cerebral NIRS, a surrogate parameter of cerebral oxygenation and perfusion. The authors concluded “that increasing cardiac output and cerebral oxygenation during apnoeic oxygenation of 15 min is a solid indicator of safety for this practice.”1
We aren’t really surprised by these findings are you? Cerebral oxygenation is known to be dependent on Mean Arterial Blood Pressure above and below the limits of autoregulation and the arterial partial pressure of carbon dioxide (PaCO2) and oxygen (PaO2). As discussed in several previous PAADs, most recently on April 15, 2025: https://ronlitman.substack.com/p/transcutaneous-carbon-dioxide-monitoring , I (MY) favor permissive hypercapnia (50-60 mm Hg) in most patients for precisely these reasons. The exceptions of course are patients with pulmonary artery hypertension and many patients with congenital heart disease, patents with intracranial pathology, or who cannot tolerate acidosis or oxygen unloading like patients with sickle cell anemia, and many neonates.
These patients were extensively monitored. I (MY) am curious if any of you using the LiDCO device to measure cardiac output? Or, NIRs in routine, non cardiac patients? How about continuous EEG? Send your thoughts and comments about the findings of this study, the use of apneic oxygenation and permissive hypercapnia to Myron who will post in a Friday reader response.
PS: If you need a refresher on cerebral autoregulation, take a look at this OpenAnesthesia summary: https://www.openanesthesia.org/keywords/cerebral-autoregulation/?search_term=cerebral%20autoregulation
If you are in the mood for testing your knowledge on cerebral physiology check out this quiz from the Society for Neuroscience in Anesthesiology and Critical Care:
https://www.snacc.org/wp-content/uploads/2020/07/cerebral-physiology-quiz-13.pdf
References
1. Kaiser HA, Bauer T, Riva T, et al. Carbon dioxide and cardiac output as major contributors to cerebral oxygenation during apnoeic oxygenation. Sci Rep 2024;14(1):3617. (In eng). DOI: 10.1038/s41598-023-49238-3.
2. Riva T, Greif R, Kaiser H, et al. Carbon Dioxide Changes during High-flow Nasal Oxygenation in Apneic Patients: A Single-center Randomized Controlled Noninferiority Trial. Anesthesiology 2022;136(1):82-92. (In eng). DOI: 10.1097/aln.0000000000004025.
3. Schwartz AE, Sandhu AA, Kaplon RJ, et al. Cerebral blood flow is determined by arterial pressure and not cardiopulmonary bypass flow rate. Ann Thorac Surg 1995;60(1):165-9; discussion 169-70. (In eng).