Transcutaneous carbon dioxide monitoring in children undergoing rigid bronchoscopy
Myron Yaster MD, Francis Veyckemans MD, Melissa Brooks Peterson, and Britta S von Ungern-Sternberg MD, PhD
First a pop quiz: If given the choice intraoperatively would you choose to ventilate your patient to an End-Tidal CO2 of 50-60 mm Hg or 30-35 mm Hg? Question 2: If one isn’t euPhemic (I know I just made up this word, but you get the idea) what is the more tolerated physiologic state: alkalosis or acidosis?
For those of you know me, I have long supported permissive hypercapnia in my anesthetized patients.1 Why? Hypercapnia produces acidosis and increases heart rate, blood pressure, and cardiac output. More importantly, it increases blood flow to the brain, heart and other organs at risk of perioperative ischemia. By increasing blood flow to the brain, it hastens the induction of anesthesia, and because CO2 (and acidosis) is the prime driver of ventilation, hypercapnia forces patients to breathe more quickly at the end of a case. Further, CO2 shifts the oxygen dissociation curve to the right and thereby helps unload oxygen throughout the body. Finally, alkalosis is almost never normal, and acidosis is common. Indeed, it happens to all of us when we exercise. Are there exceptions to this thinking? Of course! Hypercapnia can be catastrophic in patients with pulmonary artery hypertension and many patients with congenital heart disease, or with intracranial pathology, or who cannot tolerate acidosis or oxygen unloading like patients with sickle cell anemia. It probably isn’t very good in many neonates as well (although hypocapnia is much more dangerous in neonates!). Finally, although I think hypercapnia is the ideal, I aim for End tidal CO2 50-60 mm Hg, and most importantly, hypoxemia is never acceptable. Fortunately, one can have hypercapnia and normoxia by adjusting the FiO2 and end expired pressure; thus, one can have one’s cake and eat it too!
In today’s PAAD, Bordini et al.2 used transcutaneous carbon dioxide (TcCO2) monitoring in patients undergoing rigid bronchoscopy and discovered, not surprisingly, that most patients were hypercapnic during the procedure, and some developed extreme hypercarbia (TcCO2 > 90)! In today’s PAAD we will review TcCO2 and this study’s findings and implications in greater detail. Myron Yaster MD
Original article
Bordini M, Olsen JM, Siu JM, Macartney J, Wolter NE, Propst EJ, Matava CT. Transcutaneous carbon dioxide monitoring in children undergoing rigid bronchoscopy: a prospective blinded observational study. Can J Anaesth. 2025 Feb;72(2):273-284. English. doi: 10.1007/s12630-024-02862-7. Epub 2024 Oct 16. PMID: 39414716.
Transcutaneous carbon-dioxide monitors use heated skin sensors that increase skin blood flow through the cutaneous capillary system. The locally produced PCO2 is then measured electrochemically and adjusted to provide an output reflective of the arterial blood PCO2.3 Transcutaneously measured carbon dioxide (PtcCO2) has been used extensively in NICUs and PICUs with good agreement with PaCO2.2-4 Perhaps not surprisingly, Dr. Joe Tobias, one of the most prolific pediatric anesthesia researchers and writers (he has almost 1,000 publications!), has written extensively about the use of PtcCO2 perioperatively.5 For those of you who want to take a deeper dive into the technical aspects of CO2 monitoring and its many perioperative uses his review article as well as the one by Humphreys et al.6 are wonderful resources.5,6 Finally, many years ago, TCO2 monitoring fell into disfavor because of problems with calibration, differences in measurement values based on where the monitor was placed,7 and the heating element sometimes burned the skin. Limiting the heating element to a maximum of 42 degrees and the time allowed on the skin to just a few hours has mostly eliminated this latter problem.
“Rigid bronchoscopy and suspension laryngoscopy are common diagnostic and interventional procedures for a variety of pediatric airway conditions involving the supraglottis, glottis, subglottis, or proximal trachea. The anesthetic management of rigid bronchoscopy or suspension laryngoscopy is often challenging. Suboptimal ventilation states may occur because of the difficulty of providing adequate anesthesia depth for the procedure while maintaining spontaneous breathing. Intraprocedural complications may arise secondary to the anesthetic or the procedure itself, which may include hypoxemia, hypotension, apnea, or bleeding. A further challenge is ensuring adequate ventilation and anesthetic monitoring, given that, for most cases, appropriate end-tidal CO2 (EtCO2) monitoring is not achievable because of expiratory airway leakage or the need for specialized equipment (i.e., bronchoscopes with additional channels that allows the attachment of a side stream gas sampling port).”2
In today’s PAAD, Bordini et al. “sought to record TcCO2 trends in children undergoing rigid bronchoscopy/suspension laryngoscopy under general anesthesia with propofol and remifentanil. Importantly, the OR team caring for the child was blinded to the TcCO2 values so as to not influence care. Given the correlation between TcCO2 and PaCO2 values, we aimed to assess the incidences of hypercapnia (TcCO2 [ 50 mm Hg) and severe hypercapnia (TcCO2[90 mm Hg) during these procedures.”2
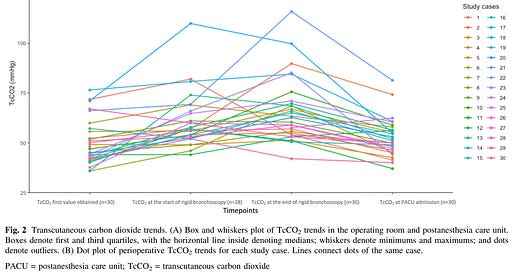
What did they find? “A total of 30 patients were enrolled. The median [interquartile range (IQR)] age was 3.5 [1.5–8.0] yr. The incidence of hypercapnia was 100% in the OR and 60% in the PACU. Five cases (17%) presented with severe hypercapnia in the OR. The highest median [IQR] TcCO2 was 69 [61–79] mm Hg. (figure) The most common adverse event was oxygen desaturation (57%, 17/30). Patients with severe hypercapnia had long stays in the PACU.”2 Most importantly, despite the hypercapnia and transient hypoxemia, “there were no serious adverse events, and all patients recovered without sequelae.”
This study also demonstrates that severe hypercapnia went clinically undiagnosed by an experienced anesthesia team. This probably means that severe hypercapnia could have been avoided if the OR team had been able to adapt ventilation or depth of anesthesia. I (FV) have the same experience when using jet ventilation in infants undergoing upper airway surgery: severe hypercapnia can occur despite apparent clinically optimal conditions and diagnosing it results in changes in ventilation settings (decrease rate or increase working pressure). We think monitoring TcCO2 should be recommended for all major airway procedures in which EtCO2 is not applicable.
Thus, from my (MY) perspective, I disagree with the authors fear of hypercapnia. I (MY) think intraoperative hypercapnia can be a useful tool in many patients but not in patients with congenital heart disease, particularly those with pulmonary artery hypertension, many neonates, and patients with intracranial pathology. The patients in this study who required postoperative stays fell into that later category.
Do you have experience with transcutaneous CO2 monitoring? Do you use it in your anesthetic (or ICU or pain) practice? Will today’s PAAD change your practice? Send your thoughts and comments to Myron who will post in a Friday reader response.
References
1. Yaster M, Gross JB. CO2 is Good for You! Anesthesia and analgesia 2021;132(1):e13. (In eng). DOI: 10.1213/ane.0000000000005270.
2. Bordini M, Olsen JM, Siu JM, et al. Transcutaneous carbon dioxide monitoring in children undergoing rigid bronchoscopy: a prospective blinded observational study. Canadian journal of anaesthesia = Journal canadien d'anesthesie 2025;72(2):273-284. (In eng). DOI: 10.1007/s12630-024-02862-7.
3. Mukhopadhyay S, Maurer R, Puopolo KM. Neonatal Transcutaneous Carbon Dioxide Monitoring--Effect on Clinical Management and Outcomes. Respiratory care 2016;61(1):90-7. (In eng). DOI: 10.4187/respcare.04212.
4. Setar L, Lee JG, Sanchez-Pinto LN, Coates BM. Accuracy and Interpretation of Transcutaneous Carbon Dioxide Monitoring in Critically Ill Children. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies 2024;25(9):e372-e379. (In eng). DOI: 10.1097/pcc.0000000000003564.
5. TOBIAS JD. Transcutaneous carbon dioxide monitoring in infants and children. Pediatric Anesthesia 2009;19(5):434-444. DOI: https://doi.org/10.1111/j.1460-9592.2009.02930.x.
6. Humphreys S, Schibler A, von Ungern-Sternberg BS. Carbon dioxide monitoring in children-A narrative review of physiology, value, and pitfalls in clinical practice. Paediatric anaesthesia 2021;31(8):839-845. (In eng). DOI: 10.1111/pan.14208.
7. Humphreys S, von Ungern-Sternberg BS, Skowno J, et al. High-flow oxygen for children's airway surgery: randomised controlled trial protocol (HAMSTER). BMJ Open 2019;9(10):e031873. (In eng). DOI: 10.1136/bmjopen-2019-031873.