Why is Fentanyl Different than other Mu agonist opioids?
Myron Yaster MD and Vidya Chidambaran MD, MS
I started my academic career in pediatric anesthesiology and critical care medicine studying fentanyl in animals (lambs) and humans (neonates) as a method of producing anesthesia and intense analgesia perioperatively. At that time (1970s-80s), human newborns were not routinely anesthetized for surgery because even in the hands of experts it was simply too dangerous.1-3 My discoveries, along with those of many others, that fentanyl could produce intense intraoperative analgesia changed how we provided anesthesia to the newborn. I succeeded because I was extremely fortunate to have the late Dr. Richard (“Dick”) Traystman as a laboratory (and professional) mentor for the animal studies and the late Dr. Theodore (“Ted”) Stanley as an advisor on the interpretation of the clinical and laboratory findings.4 (It is beyond the scope of today’s PAAD to review Dr. Stanley’s history of fentanyl but I would highly recommend it! See in references)
In these early studies I discovered that fentanyl behaved differently than other mu-opioid agonists. It produced chest wall rigidity and morphine did not and the chest wall rigidity appeared to precede or was independent of ventilatory depression. The lambs required a lot of fentanyl, mg/kg, not micrograms/kg (and hence the development of carfentanil for large animal veterinary medicine.) Finally, it produced intense analgesia but not anesthesia. Indeed, the lambs were always awake and responded to sound even when they had no response to intense pain. I asked Dr. Stanley “Why”? To be honest, I never did get a good explanation and have always wondered “why”? In today’s PAAD, Kelly et al.5 provide some insights and explanations based on the molecular biology of how fentanyl binds to the mu opioid receptor to provide some of the answers. Further, these insights also explain why we use fentanyl in our clinical practice and why, when fentanyl is abused, why it is so frequently lethal.
Volume 180 issue 7 of the British Journal of Pharmacology is a themed issue on advances in opioid pharmacology at the time of the opioid crisis. Over the next couple of weeks the PAAD team will be reviewing several of these papers. First up will be a paper by Kelly et al.5 on why fentanyl and its derivatives, including designer fentanyls, are associated with so many overdose deaths. What are the properties of fentanyl that may differentiate it from other μ agonists? Next week we’ll discuss a laboratory study of why the combination of benzodiazepines AND OXYCODONE is often lethal and in 2 weeks another paper that discusses the mechanisms of opioid-induced respiratory depression. We’ll also review an article on how opioid receptors work and how this knowledge is being used to develop new targets of drug development.
I’ve asked Dr. Vidya Chidambaran of Cincinnati Children’s Hospital and one of our profession’s brightest stars and leading researchers in perioperative pain and opioid safety to assist on this and some other articles in this themed British J of Pharmacology issue. I can’t do justice to all of Vidya’s accomplishments and refer you to her lab’s webpage for more information on her current research. Myron Yaster MD
https://www.cincinnatichildrens.org/research/divisions/a/anesthesia/labs/chidambaran
Original article
Eamonn Kelly, Katy Sutcliffe, Damiana Cavallo, Nokomis Ramos-Gonzalez, Norah Alhosan, Graeme Henderson. The anomalous pharmacology of fentanyl. Br J Pharmacol. 2023 Apr;180(7):797-812. PMID: 34030211
We don’t have to tell you that fentanyl and its related “designer” congeners, alfentanil, sufentanil, and remifentanil, are the building blocks of balanced general anesthesia and how fentanyl in particular is used in virtually all modern anesthetics. All of you use it in your everyday practice and understand how terrific this drug is perioperatively. After all, the fentanyls are extremely potent, rapidly acting, intensely analgesic, and short acting, making them perfect for what Dr. Peter Davis of the University of Pittsburgh calls “rawhide anesthesia”, that is, “move ‘em in, move ‘em out” anesthesia. Unfortunately, many of these same properties have made illicitly manufactured fentanyl THE primary opioid of abuse in the United States today. Indeed, it has largely replaced heroin. In fact, as discussed in a recent PAAD, in 2022, fentanyl was responsible for more than 80,000 overdose deaths in the United States.6
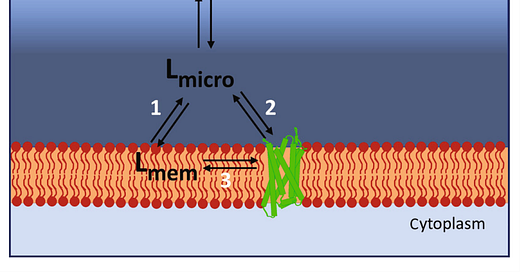
The question the authors address in the paper referenced is whether fentanyl behaves pharmacologically in ways that are different from other mu receptor agonists such as morphine or oxycodone. As we know, they are all bind to the same mu-opioid (G-protein coupled) receptor but don’t have similar lethal potentials. What accounts for this? Let’s take a close look at this schematic cartoon reproduced below illustrating increased local ligand or agonist concentration in the vicinity of the cell membrane and pathways for ligand binding to the receptor: Pathway 1—ligand entering the lipid membrane; Pathway 2—ligand binding to the receptor via the aqueous route; and Pathway 3—ligand diffusing into the orthosteric binding pocket of the receptor from the lipid.
When fentanyl was first developed by Dr. Paul Janssen in 1963, it was designed to be fast acting, potent, and short acting by making the parent molecule (meperidine) highly lipophilic.4 This allowed fentanyl to rapidly cross the blood brain barrier and to achieve its effect via paths 1,2, and 3. Less lipophilic drugs like morphine achieve their effects only by pathway 2. The higher lipid solubility of fentanyl compared with morphine will mean that more fentanyl may enter the cell membrane, thus leading to higher concentrations of fentanyl than morphine around the μ receptor (pathway 3), even though the concentration of these drugs in the general bathing medium was the same. In addition, fentanyl molecule has a central nitrogen of its piperidine ring, along with an elongated and flexible structure that seems to allow it to adopt different orientations (poses) in the mu receptor while maintaining GPCR activations, thus providing an advantage of receptor occupation efficacy over morphine and other ligands. Its molecular flexibility and lipophilicity also allows it a unique ability to maneuver between transmembrane domains (6 and 7) of the receptor to reach the binding pocket, while the morphine molecule is unable to do so. Hence, superior accessibility to the GPCR seems to contribute to its superior efficacy (at smaller doses) and its lethal potential.
In last week’s PAAD, we discussed oliceridine, the first of the biased ligand opioids which theoretically will produce less respiratory depression and tolerance compared to standard opioids like morphine. When most opioids bind to the mu receptor (path 2 in figure), they activate the G-coupled receptor pathway. The activated phosphorylated cytosolic portion of the receptor then attracts a beta arrestin molecule which essentially turns the receptor off. Further, this beta arrestin activated complex essentially destroys the receptor and thereby produces tolerance, a property that we routinely see with fentanyl particularly when it is given by continuous infusion. Fentanyl was previously thought to be arrestin biased, but more recent research suggest it is not. In fact, recent research also shows that fentanyl likely induced less tolerance and very little cross-tolerance with other opioids such as morphine. This differential tolerance to respiratory depression could be a crucial factor in fentanyl overdose deaths.
Perhaps the most lethal and unusual property of fentanyl is the production of chest wall (muscle) rigidity. This can occur whenever fentanyl is given rapidly IV as a bolus. No one is quite sure how this happens but is thought to occur by the rapid entrance of fentanyl into the brain. If you’ve ever experienced chest wall rigidity in one of your patients you will never forget it. I (MY) was involved in the original remifentanil studies by Drs. Jeff Galinkin, Peter Davis, and others7, 8 In those study protocols, I gave a rapid IV bolus of remifentanil to a newborn. To be honest what happened next scared the bejesus out of me. I could neither oxygenate nor ventilate the patient. It was likely trying to ventilate a bag of cement. Naloxone and pancuronium broke the rigidity but not my memory of it! I’ve long suspected that many of the illicit fentanyl overdose deaths are caused by fentanyl induced upper airway obstruction and muscle rigidity and not just respiratory depression.
Another important point borne by research is that 10-fold higher doses of naloxone are required to reverse fentanyl (versus morphine), which might also be contributing to ease of over “over-dosing” on fentanyl!
PS: Dr Paul Janssen the inventor of fentanyl also synthesized and created 80 other drugs including diphenoxylate (Lomotil), haloperidol (Haldol), and droperidol to name a few. Interestingly, in light of the current tsunami of illicit fentanyl overdose deaths, it’s worth noting that the FDA and its consultant, the legendary Dr. Robert Dripps of the University of Pennsylvania, opposed the approval of fentanyl because of its abuse potential and insisted that it be sold only in combination with droperidol (Innovar)!.4
References
1. Yaster M, Koehler RC, Traystman RJ. Effects of fentanyl on peripheral and cerebral hemodynamics in neonatal lambs. Anesthesiology. 1987 1987;66(4):524-530. Not in File.
2. Yaster M. The dose response of fentanyl in neonatal anesthesia. Anesthesiology. 1987 1987;66(3):433-435. Not in File.
3. Yaster M. Analgesia and anesthesia in neonates. JPediatr. 1987 1987;111(3):394-396. Not in File.
4. Stanley TH. The fentanyl story. The journal of pain : official journal of the American Pain Society. Dec 2014;15(12):1215-26. doi:10.1016/j.jpain.2014.08.010
5. Kelly E, Sutcliffe K, Cavallo D, Ramos-Gonzalez N, Alhosan N, Henderson G. The anomalous pharmacology of fentanyl. British journal of pharmacology. Apr 2023;180(7):797-812. doi:10.1111/bph.15573
6. Kuehn BM. Fentanyl Drives Startling Increases in Adolescent Overdose Deaths. Jama. 2023;329(4):280-281. doi:10.1001/jama.2022.23563
7. Galinkin JL, Davis PJ, McGowan FX, et al. A Randomized Multicenter Study of Remifentanil Compared with Halothane in Neonates and Infants Undergoing Pyloromyotomy. II. Perioperative Breathing Patterns in Neonates and Infants with Pyloric Stenosis. AnesthAnalg. 12/2001 2001;93(6):1387-1392. Not in File.
8. Davis PJ, Galinkin J, McGowan FX, et al. A randomized multicenter study of remifentanil compared with halothane in neonates and infants undergoing pyloromyotomy. I. Emergence and recovery profiles. AnesthAnalg. 12/2001 2001;93(6):1380-1386. Not in File.