To transfuse or not transfuse in pediatric Sickle Cell Disease that is the question
Myron Yaster MD and Lynne G. Maxwell MD
In the very early days of SPA, well before the development of the quality and safety committees and multi-institutional clinical trials groups like the pediatric difficult airway registry, Dr. Sal Goodwin of the University of Florida and the Nemours clinic in Jacksonville, Florida, Dr. Tom Mancuso of Boston Children’s Hospital, Dr. Paul Firth of Massachusetts General Hospital, Dr. Chuck Haberkern of the Seattle Children’s Hospital and several others formed one of the very first Special Interest Groups (SIGs) in SPA which was devoted to improving the care of patients with sickle cell disease (SCD) using the best evidence based knowledge available at the time.1-3 The SIG disbanded when the members felt that consensus had been reached and no further efforts were needed. Nevertheless, the optimization of the pediatric patient with sickle cell disease (SCD) continues to be an area of interest, given the incidence and the perioperative complications of the disease.4
In today’s PAAD, published in the Anesthesia Patient Safety Foundation newsletter, Baijal et al. 5 revisit perioperative transfusion practices in children with sickle cell disease. While preparing today’s PAAD, I found a Power point lecture prepared by Dr. Andrew Infosino on the Society for Pediatric Anesthesia’s lecture series website. For those of you who teach or present grand rounds on this topic, Dr. Infosino’s lecture is terrific and has simply beautiful figures that I think you can “borrow”. Finally, we’ve covered this topic in a previous PAAD on 06/05/2023 that you may want to download and also review. https://ronlitman.substack.com/p/perioperative-management-of-patients Myron Yaster MD
Original article
Baijal RG, Dalal PG, Kanjia M. Preoperative Transfusion and Sickle Cell Disease in the Pediatric Patient. Anesthesia Patient Safety Foundation. 06/2024
Electronic media
Infosino, A: Anesthetic Considerations for Pediatric Patients With Sickle Cell Disease. SPA Powerpoint lecture series, presented by the SPA Global Committee https://pedsanesthesia.org/education-and-meetings/powerpoint-lecture-series/
“Sickle cell disease is a common hematologic defect with a substitution of valine for glutamic acid on the beta chain of hemoglobin, occurring in about 1 out of 365 African American births. The red blood cells (RBC) in these patients, when deoxygenated, undergo polymerization leading to RBC deformity (i.e., sickling), subsequent hemolysis, and vaso-occlusion. This RBC damage, precipitated by hypoxemia, hypothermia, hypovolemia, infection, pain, stress, and surgery can inhibit blood flow and cause ischemic injury, producing the symptoms of a sickle cell crisis, such as a pain crisis, acute chest syndrome, chronic organ damage, stroke, priapism, and musculoskeletal complications. Children with SCD are at an increased risk for the following postoperative complications, with the incidence of an acute chest syndrome (ACS) of 3.08%, stroke of 0.2%, and 30-day mortality of 0.2%.”5,6 Patients with beta thalassemia (Hb-S-beta0 are similarly vulnerable to these postoperative complications.
For the past 20+ years a consensus has developed of how to prepare a patient with SCD for surgery. First, always, and we do mean always, plan on consulting the patient’s hematologist well in advance of the day of surgery to plan your course of action. Intraoperatively, surgery may lead to hypotension, acidosis, hypoxia, exposure to low temperatures, vasoconstriction, venous stasis, and increased stress, all of which can promote increased sickling and potentially induce vaso-occlusive episodes (VOEs). Additionally, patients with SCD have higher risks of perioperative infection due to functional asplenia, and increased risk of perioperative thrombosis as a result the underlying hypercoagulable state. Preoperative planning is required to help minimize the risks of perioperative morbidity and mortality.4
Preoperative transfusion is the key to therapy and may reduce the perioperative risk of acute chest syndrome and stroke even in low to medium risk surgical procedures like tonsillectomy.3,7 How much to transfuse? Controlled studies found no difference in complications between patients who received “aggressive transfusion” defined as targeting a hemoglobin of 10 g/dL and HbS <30% via exchange transfusion, and those who received “simple transfusion” defined as targeting a hemoglobin of 10 g/dL irrespective of HbS percentage.3 Thus, simple transfusion to a post transfusion hemoglobin concentration of 10g/dL is the way most folks treat these patients.8 A more recent study evaluated data from the American College of Surgeons NSQIP (National Surgical Quality Improvement Program) pediatric database. A retrospective cohort of 357 children with SCD who underwent low to moderate risk surgery (laparoscopic cholecystectomy, splenectomy, or appendectomy) revealed no difference in multiple outcomes including 30-day readmission rates, pneumonia, venous thromboembolism, postoperative transfusion, cardiac arrest, stroke, sepsis and death between children who were transfused preoperatively versus those who were not transfused. Subgroup analysis of those with a preoperative hematocrit > 27.3% or less than 27.3% showed no difference in postoperative sickle cell crisis in those children who were transfused versus those who were not transfused. In addition, preoperative transfusion was not associated with a reduced rate of postoperative transfusions.9 It should be emphasized that the severity of SCD was not characterized and the surgeries were low to moderate risk.
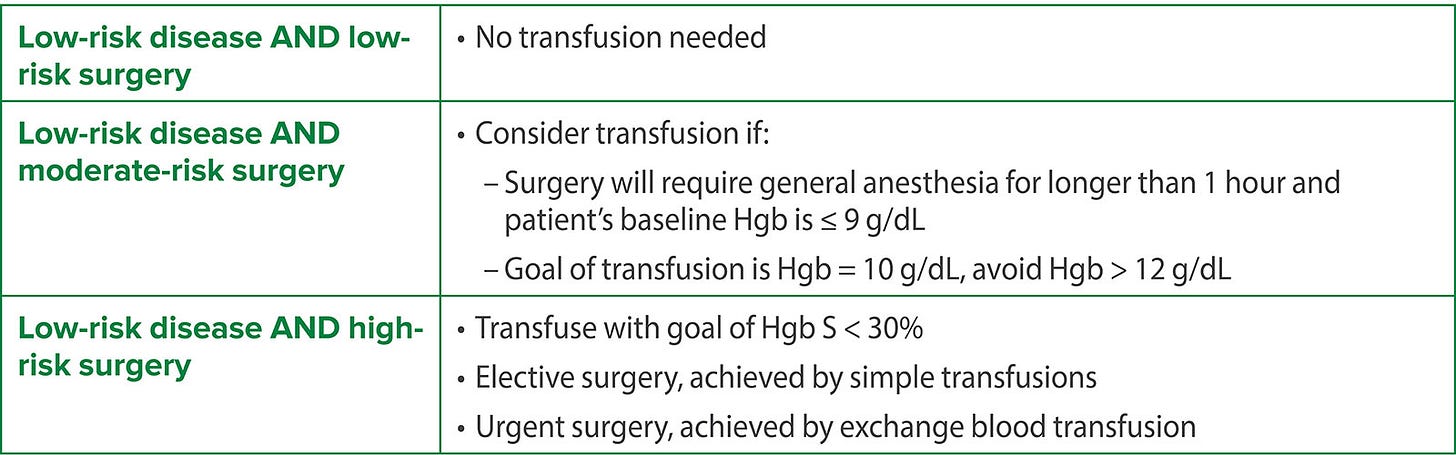
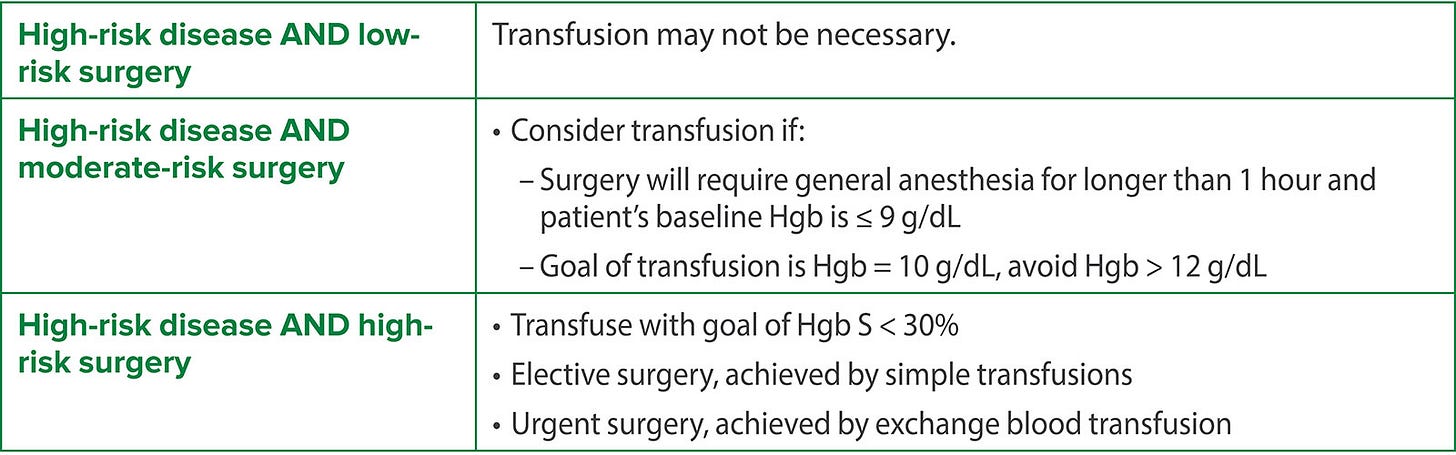
So does every patient with SCD really need to be transfused preoperatively? Or to paraphrase the Bard: To transfuse or not to transfuse; that is the question. Baijal et al.5 suggest that the decision should be guided by disease severity and the surgery category. Patients who may benefit from transfusion are patients at high risk for decompensation and include those who are either undergoing a high-risk procedure or at baseline have a high-risk disease state. “The most common pediatric procedures are low-to-moderate risk (e.g. pressure equalizing tube insertion, laparoscopic cholecystectomy, tonsillectomy/adenoidectomy, laparoscopic splenectomy, umbilical hernia repair, laparoscopic appendectomy, and myringotomy tubes) as compared to adults who may undergo more high-risk procedures (e.g., cardiac surgery and cerebral revascularization.”5 Patients with severe SCD have had > 2 acute chest syndrome events in the past 5 years, have had a history of stroke, and/or have asthma or abnormal renal function. Some patients with high-risk SCD who have had strokes or frequent acute chest syndrome may be on a chronic transfusion regimen. These patients may not need additional transfusion if their Hb is > 10 g/dL (although their transfusion schedule may need to be adjusted to be closer to the time of scheduled surgery).
This is summarized by the following tables.
TCD = Transcranial Doppler
Regardless of whether preoperative transfusion is administered, intraoperative management should be optimized to reduce the risk of VOE. These prophylactic measures include liberal preoperative and intraoperative intravenous fluid administration, warming measures to maintain normothermia, and maintenance of normoxia. The risk for vaso-occlusive complications associated with the use of a tourniquet in orthopedic surgery is a controversial topic, sometimes said to be contraindicated in patients with SCD. Pignatti et al addressed this issue in a literature review published in the hematology literature in 2017. 10 They concluded “From what we could find in the literature and contrary to what is suggested by most guidelines it appears that complications are rare. However, caution must be applied and the risk/benefit ratio carefully considered.”
What are your thoughts? Send your comments to Myron who will post in a Friday reader response.
References
1. Firth PG, McMillan KN, Haberkern CM, Yaster M, Bender MA, Goodwin SR. A survey of perioperative management of sickle cell disease in North America. PaediatrAnaesth 2011;21(1):43-49.
2. Goodwin SR, Haberkern C, Crawford M, Lerman J, Mancuso T, Yaster M. Sickle cell and anesthesia: do not abandon well-established practices without evidence. Anesthesiology 2005;103(1):205-207.
3. Vichinsky EP, Haberkern CM, Neumayr L, et al. A comparison of conservative and aggressive transfusion regimens in the perioperative management of sickle cell disease. The Preoperative Transfusion in Sickle Cell Disease Study Group [see comments]. NEnglJMed 1995;333(4):206-213.
4. Azbell RCG, Lanzkron SM, Desai PC. Current Evidence and Rationale to Guide Perioperative Management, Including Transfusion Decisions, in Patients With Sickle Cell Disease. Anesthesia and analgesia 2023;136(6):1107-1114. (In eng). DOI: 10.1213/ane.0000000000006463.
5. Baijal RG, Dalal PG, Kanjia M. Preoperative Transfusion and Sickle Cell Disease in the Pediatric Patient. Anesthesia Patient Safety Foundation. 06/2024 (https://www.apsf.org/article/preoperative-transfusion-and-sickle-cell-disease-in-the-pediatric-patient/?utm_source=APSF+Newsletter+Email+-+External&utm_medium=email&utm_campaign=EMAIL_CAMPAIGN_2024_06_01).
6. Hyder O, Yaster M, Bateman BT, Firth PG. Surgical procedures and outcomes among children with sickle cell disease. Anesthesia and analgesia 2013;117(5):1192-6. (In eng). DOI: 10.1213/ANE.0b013e3182a44d74.
7. Waldron P, Pegelow C, Neumayr L, et al. Tonsillectomy, adenoidectomy, and myringotomy in sickle cell disease: perioperative morbidity. Preoperative Transfusion in Sickle Cell Disease Study Group. JPediatrHematolOncol 1999;21(2):129-135.
8. Chou ST, Alsawas M, Fasano RM, et al. American Society of Hematology 2020 guidelines for sickle cell disease: transfusion support. Blood Adv 2020;4(2):327-355. (In eng). DOI: 10.1182/bloodadvances.2019001143.
9. Salvi PS, Solomon DG, Cowles RA. Preoperative Transfusion and Surgical Outcomes for Children with Sickle Cell Disease. J Am Coll Surg 2022;235(3):530-538. (In eng). DOI: 10.1097/xcs.0000000000000267.
10. Pignatti M, Zanella S, Borgna-Pignatti C. Can the surgical tourniquet be used in patients with sickle cell disease or trait? A review of the literature. Expert Rev Hematol 2017;10(2):175-182. (In eng). DOI: 10.1080/17474086.2017.1273765.