The Never Ending Quest To Achieve Hemostasis: At What Cost?
Susan Nicolson MD, Lindsey Loveland Baptist MD, Viviane Nasr MD, James DiNardo MD
Original article
Manchula Navaratnam, Julianne M Mendoza, Shiqi Zhang, Derek Boothroyd, Katsuhide Maeda, Komal Kamra, Glyn D Williams. Activated 4-Factor Prothrombin Complex Concentrate as a Hemostatic Adjunct for Neonatal Cardiac Surgery: A Propensity Score-Matched Cohort Study. Anesth Analg. 2023 Mar 1;136(3):473-482. PMID: 36729967
Editorial
David Faraoni, Roman M Sniecinski. FEIBA Use in Neonatal Cardiac Surgery: A Risky Business That Needs Further Investigation. Anesth Analg. 2023 Mar 1;136(3):470-472. PMID: 36806234
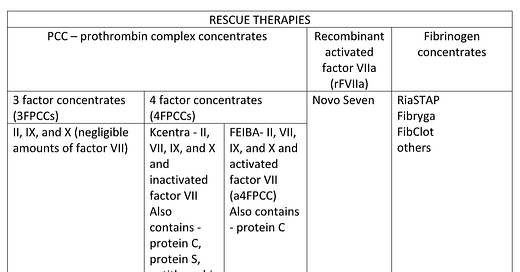
Neonates undergoing cardiac surgery requiring bypass often develop coagulopathy resulting in major postoperative blood loss. Risk factors that increase post-bypass bleeding in neonates include: immature hemostatic systems, low levels of coagulation factors, low weight, long hypothermic bypass times, cyanotic congenital heart disease and imperfect suture lines. Increased post-bypass bleeding and donor exposures are independently associated with adverse outcomes. Bleeding management routinely consists of transfusion of component therapy, assuming fresh whole blood is unavailable, ideally guided by rapid turnaround testing. When transfusion of hemostatic blood products fails to reduce the bleeding to an acceptable level (varies from practitioner to practitioner), rescue therapies, including prothrombin complex concentrates (PCCs), and/or recombinant activated factor VII (rFVIIa) are being given “off-label” with increasing frequency (see chart). Neonates are at increased risk for postoperative thromboembolism without the addition of PCCs or rFVIIa. Risk factors include: cyanosis, blood product transfusion, long bypass times, use of deep hypothermic circulatory arrest and indwelling central venous catheters. The limited literature on using these agents in neonates is most commonly retrospective, observational, performed in a single center with varying doses and timing of administration in a small number of patients resulting an inability to determine efficacy or safety.
PCCs are formulations containing vitamin K-dependent clotting factors. Ex vivo studies show that Vitamin K-dependent clotting factors play a role in thrombin generation and clot formation. PCC are classified as 4-factor prothrombin complex concentrates (4FPCCs) containing factors II, VII, IX and X and 3-factor (3FPCCs) prothrombin complex concentrates containing factors II, IX and X. 4FPCCs are further differentiated according to whether they contain activated factor VII (FEIBA) or inactivated (Kcentra). Both these agents are available in the US. Further complicating the picture is the presence of protein C, protein S, antithrombin 3 (AT3), and heparin in Kcentra while FEIBA only additionally contains protein C. This raises an interesting decision-making process when Kcentra or FEIBA is considered for treatment of residual bivalirudin anticoagulation in the setting of heparin induced thrombocytopenia with thrombosis (HITT).
FEIBA is a standard bypassing agent created and approved for management of bleeding episodes in patients with hemophilia with inhibitors, not for use in neonates with acquired coagulopathy. Because FEIBA contains the activated form of factor VII and small amount of activated factor X there have been concerns, including an FDA package insert warning, regarding the potential for thrombotic events. The incidence of thrombotic complications appears dose dependent and is higher when given after bypass compared to the intended use.
The paper by Navarathnam and colleagues is the first published data reporting the use of FEIBA in a large number of exclusively neonates following cardiac surgery with bypass. The electronic heath record of 165 virgin chest neonates undergoing surgery at Stanford between 2014-2018 were retrospectively reviewed. Patients were given FEIBA (10 IU/kg) if their surgeon and anesthesiologist assessed refractory bleeding (no definition, no coagulation results) post-bypass despite standard blood component therapy. Repeat doses of FEIBA were given on clinical assessment of bleeding. Of the 79 neonates who were given FEIBA, 43 were propensity score matched to neonates who did not receive FEIBA. The primary efficacy outcome was total volume of blood product (ml/kg) transfused after bypass, including the 1st 24 hours in the CICU. A statistically significant difference in mean total blood products transfused was found in the FIEBA group (48 ml/kg) compared with the control group (64 ml/kg). However, the authors were not able to demonstrate any difference in blood products transfused either from termination of bypass to leaving the operating room or the 1st 24 hours in the CICU. Administration of FEIBA as a rescue agent at a variable time post-bypass may in part explain why no difference was found in the OR epoch. No data was presented on whether there was a reduction in donor exposure.
The primary safety outcome was the incidence of 7 and 30 day postoperative thromboembolism. Although no difference in the thromboembolic rate was found at either day, the study was not powered to identify a difference and did not delineate which tools were used for surveillance. The thrombotic rates were no different between patients who received a total dose of greater or equal to 20 IU/kg compared to those who received a total dose of less than 20 IU/kg.
Secondary outcomes were the same for the 2 groups with the exception of CICU and hospital length of stays. Patients in the FEIBA groups had concerningly longer lengths of CICU stay (33 versus 12 days; P= 0.049) and hospital stay (45 versus 24 day; P=0.049). This difference points to the fact that either the variables used in the matching might have been too restrictive and/or the possibility of unmeasured, or unknown side effects from FEIBA that prolonged length of stay.
The results of this study may not have generalizability to other centers. Multi-center prospective well-designed studies are needed to determine the risk benefit of FEIBA after neonatal cardiac surgery. Until such results are available FEIBA and other procoagulants need to be used with caution, in the lowest dose that has been demonstrated to be effective (5-10 IU/kg), in a limited number of neonates with pre-identified quantified residual bleeding after appropriate and documented correction of coagulopathy with conventional hemostatic blood products. The decision to give FEIBA to a neonate following bypass needs to be made when the consequences of ongoing blood loss and replacement poses more risk than the risk of thrombotic complications from the drug in a population with a known increased risk of postoperative thromboembolism.