The AnaConDa (Anesthesia Conserving Device) Revealed
Jeffrey M. Feldman MD, MSE, FASA
In the September 10th PAAD, Inhaled isoflurane for sedation of mechanically ventilated children in intensive care, we discussed the use of the Sedaconda ACD-S device (Sedonamedical), a commercially available device in Europe that allows the use of sevoflurane for “sedation” in the PICU and overcomes the issues of high flow and scavenging in the ICU. To be honest, I’ve heard of this device but have never seen or used it and I couldn’t figure out how this device works! So I asked Dr. Jeff Feldman, the PAAD’s ventilator maven to look into this. In today’s PAAD, Dr. Feldman takes a deeper dive into this device and how it works. For those of you who have used please send me your thoughts and I will post in a Friday reader response. Myron Yaster MD
Original article
Farrell R, Oomen G, Carey P. A technical review of the history, development and performance of the anaesthetic conserving device "AnaConDa" for delivering volatile anaesthetic in intensive and post-operative critical care. J Clin Monit Comput. 2018 Aug;32(4):595-604. doi: 10.1007/s10877-017-0097-9. Epub 2018 Jan 31. PMID: 29388094; PMCID: PMC6061082.
The public demonstration of ether in 1846 showed the power of inhalation anesthesia to quickly produce hypnosis, immobility and insensibility to pain. Modern inhalation anesthetics provide these unique pharmacologic properties with a side effect profile that is easily managed by a trained clinician. In addition to the desirable combination of pharmacologic effects, the dosing convenience of inhalation anesthetics is also a completely unique feature. In contrast to every other route of drug delivery (IV, PO, PR, Transdermal), the effect site concentration of an inhalation anesthetic is easily controlled and inferred using readily available monitors. If too much is given, or the drug effect is no longer desired, it is an easy process to retrieve the drug from the body. Furthermore, end-tidal agent concentration can be monitored continuously to provide dosing guidance.
Given the unique and desirable properties of inhalation anesthetics, it is not surprising that they are attractive for use as sedative-hypnotics outside of the operating room eg. in the ICU. Anesthesia machines are specifically designed to deliver inhalation anesthetics efficiently and have been used in ICUs for sedation and other therapies such as status asthmaticus and epilepticus. However, intensivists and respiratory therapists are not necessarily familiar with using anesthesia machines and the mechanical ventilation capabilities of the anesthesia machine may not be ideal for the ICU patient. The question then becomes how to use more familiar equipment like the ICU ventilator to deliver an inhaled anesthetic agent safely and effectively. Many years ago, Siemens provided a vaporizer option for their ICU ventilator which added inhaled anesthetic to the inspired gas. While it worked, the inability to capture exhaled anesthetic agent resulted in a great deal of waste and environmental pollution. The AnaConDa was designed to enable safe and effective inhaled anesthetic delivery, and minimize waste and pollution, while using an ICU ventilator. How does it work? Is it “snake oil” or a real solution to the problem?
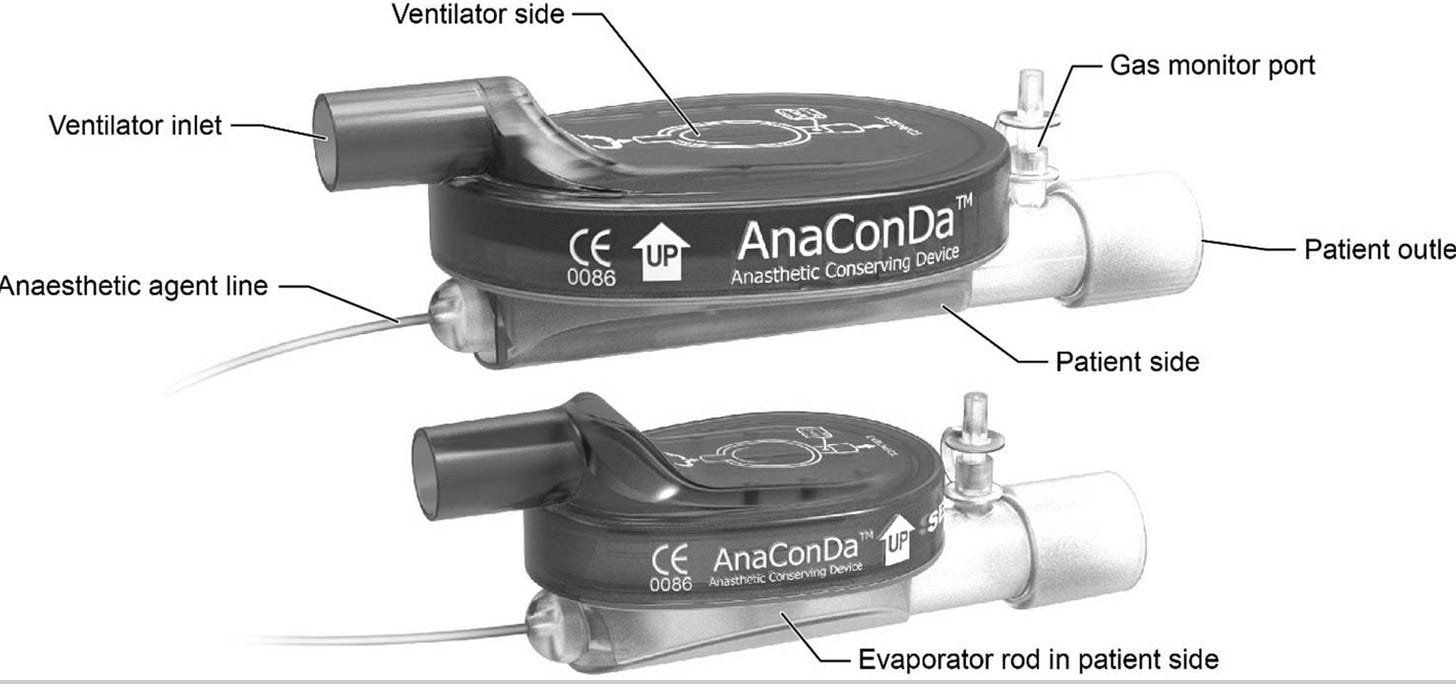
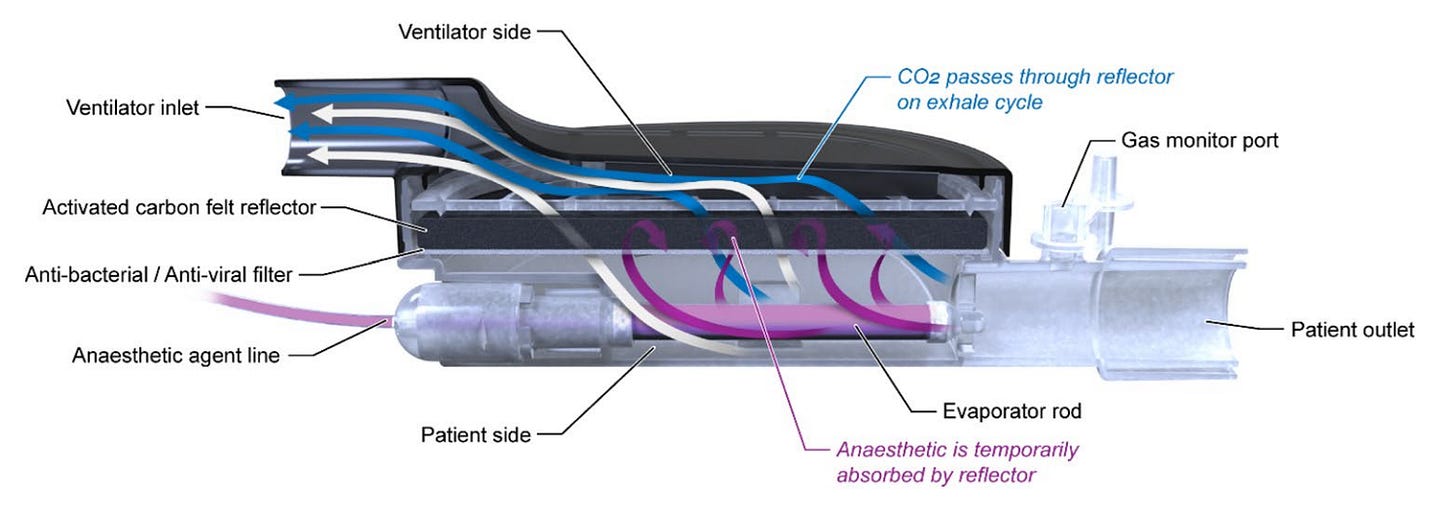
There is a thorough and excellent review of the AnaConDa that should answer most, if not all, questions about the utility of the device for using an ICU ventilator to deliver inhaled anesthetics. (1) In a nutshell, this device is essentially a heat and moisture exchanger (HME) with the capability to also capture and redeliver inhaled anesthetics. HME’s are placed between the y-piece of the ventilator circuit and the endotracheal tube. These devices include a special membrane that absorbs moisture in the exhaled gas and releases the moisture back to the patient during inspiration. They also typically include a filter for capturing infectious material – both viral and bacterial. The AnaConDa adds to the typical HME membrane another layer called an activated carbon felt reflector. Activated carbon is well known to absorb inhalation anesthetic agents and is used in other devices such as the Vapor-Clean (Dynasthetics, Salt Lake City, UT) which prevents inhaled anesthetics from reaching a patient who may be susceptible to MH. The reflector in the AnaConDa has been designed to absorb inhaled anesthetics from exhaled gas and return the drug to the patient during inspiration. The device includes a port for administering liquid anesthetic via a syringe pump and another port for measuring the concentration of anesthetic and other gases.
The review article summarizes clinical studies documenting the effectiveness of the AnaConDa for use with an ICU ventilator to provide safe and effective sedation with Isoflurane or Sevoflurane. Desflurane cannot be used since it becomes a gas (boils ) at room temperature. About 90% of the anesthetic is captured by the AnaConDa which is quite efficient, but some method is still required to connect exhaled gases leaving the ventilator to a scavenging system.
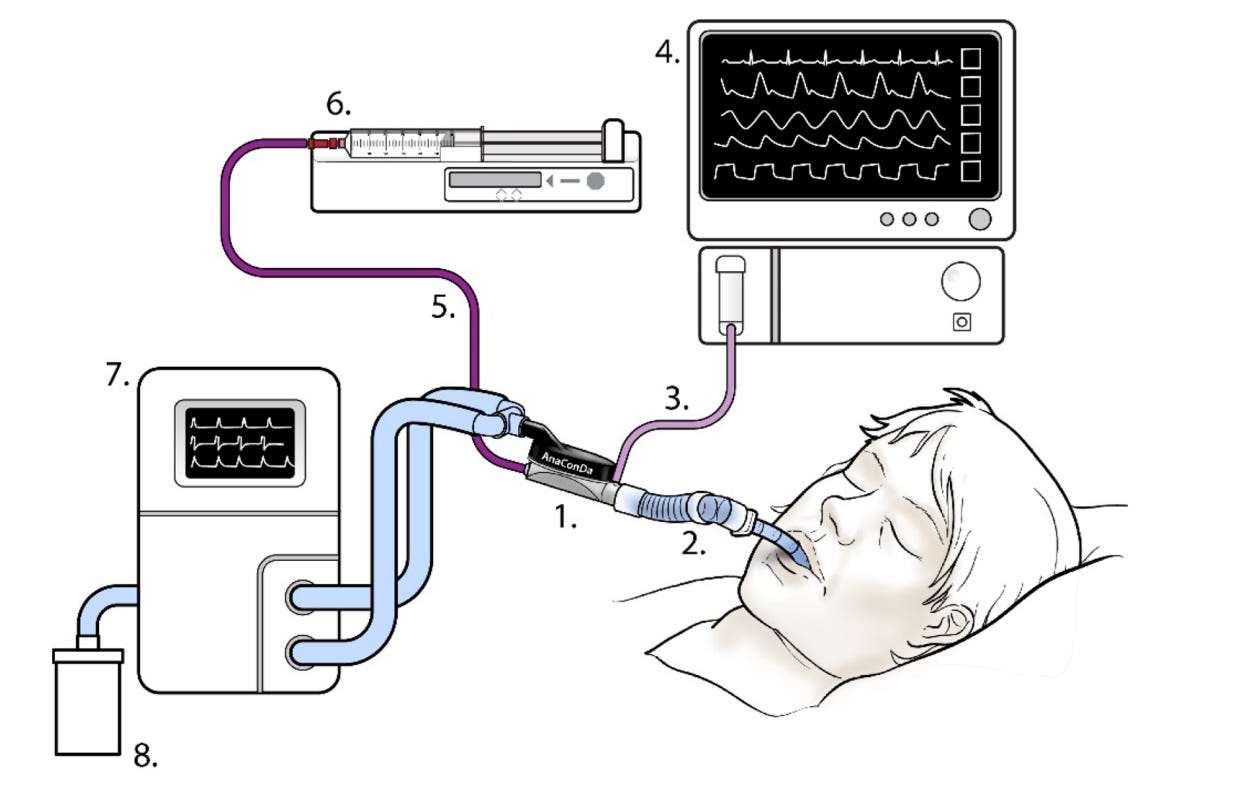
“A typical set-up with the AnaConDa is seen in Fig. 6 below. The AnaConDa (1) is installed in place of the HME filter in the ventilator circuit, connected to the Y-piece and the endotracheal tube or flex-tube, (2) the AnaConDa should be oriented at an angle, tilted slightly downward towards the patient, as shown, to prevent fluid from accumulating in it. The AnaConDa gas sampling line, (3) is plugged into a gas monitor or patient monitoring unit with gas module, (4) the sedative agent line, (5) is threaded onto a full syringe placed in the syringe pump, (6) the ventilator settings, (7) have to account for the increased dead space of the AnaConDa and flex-tube or adaptor. Scavenging can be assured by affixing a scavenging canister to the exhaust port of the ventilator or other means.”
Full disclosure, I have never used the device personally to provide “sedation”. Hopefully, this description, and the associated reference, is helpful for understanding the potential of this device to provide sedation with an inhaled anesthetic agent while using an ICU ventilator. The device is not approved by the FDA for use in the United States except as an investigational device when intravenous sedatives have failed.