Remembering the classics: The Precordial Stethoscope
Mark Rockoff MD, Alan Jay Schwartz MD MSEd, Francis Veyckemans MD
For several decades after William Morton’s 1846 demonstration of ether at the Massachusetts General Hospital, little monitoring was done by those administering what would be termed “anesthesia” (the surgeon’s response to the ether demonstrations: “this is no humbug!”). In the USA, ether was given by an orderly or trainee; in England and some other countries, chloroform was soon preferred and was given by physicians since it was more challenging to administer. Observation of the patient’s skin color and respiratory efforts, with occasionally pulse palpation, were all that were monitored, as long as the patient was not moving during the procedure. Therefore, it is not surprising that surgery was accompanied by significant morbidity and mortality. A remarkable description of conditions as they were at the time was given by Harvey Cushing (yes, that Harvey Cushing!) who recounted his experience in 1894 at Harvard Medical School, before he became a pioneer in neurosurgery.
“My first giving of an anesthetic was when, a third-year student, I was called down from the seats and sent to a little side room with a patient and an orderly and told to put the patient to sleep. I knew nothing about the patient whatsoever, merely that a nurse came in and gave the patient a hypodermic injection. I proceeded as best I could under the orderly’s directions, and in view of the repeated urgent calls for the patient from the surgical amphitheatre it seemed to be an interminable time for the old man, who kept gagging, to go to sleep. We finally wheeled him in. I can vividly recall just how he looked and the feel of his bedraggled whiskers. The operation was started and at this juncture there was a sudden great gush of fluid from the patient’s mouth, most of which was inhaled, and he died. I stood aside, burning with chagrin and remorse. No one paid the slightest attention to me, although I supposed that I had killed the patient. To my perfect amazement I was told it was nothing at all, that I had nothing to do with the man’s death, that he had a strangulated hernia and had been vomiting all night anyway, and that sort of thing happened frequently and I had better forget about it and go on with the medical school. I went on with the medical school, but I have never forgotten about it.” [1]
Partly because of this experience, Cushing always maintained a great interest in anesthesia. Soon thereafter, he and a fellow student introduced anesthesia record keeping for pulse and respiratory rates. Indeed, his charting at 5-minute intervals on graph paper is virtually the same as we use today. A few years later, as a young surgeon on the staff at the Johns Hopkins, he advocated for routine intraoperative blood pressure monitoring and the continuous use of a precordial stethoscope (then called a phonendoscope).
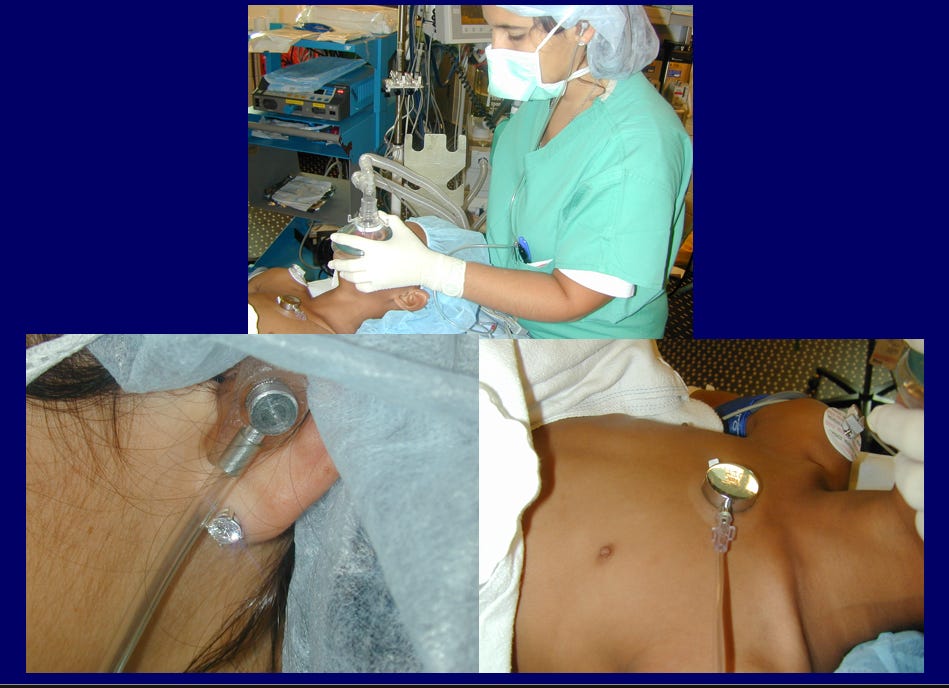
“We have, of late, in all of our cerebral operations, followed the custom of having the etherizer constantly auscultate the heart. This is accomplished by strapping the transmitter of a phonendoscope to the precordium. From this a long tube passes to the aural receiver which is held against the auricle as is the receiver of a telephone operator. This is much more satisfactory than the usual supervision of cardiac action by the occasional palpation of the pulse for which a hand must be disengaged. It is surprising that the method has not come into general practice before this.”[2] [3]
Actually, the history of the stethoscope is quite interesting and worth a brief historical detour. The word itself is derived from the Greek “stethos” (“chest”) and “skopein” (“view/examine”). It was first developed in 1816 by Rene’ Laennec, a French physician who used a simple wooden tube to avoid having to place his ear directly on a women’s chest to listen to her heart. A binaural version was created in 1851 by the Irish physician, Arthur Leared, improved the following year by the American George Cammann, and several modifications were subsequently developed. By the 1890s, rubber tubing had been widely incorporated into the stethoscope design improving its flexibility and making it more practical and comfortable. In 1894 two Italian physicians, Eugenio Bazzi and Aurelio Bianchi, introduced a binaural stethoscope which amplified sounds and became known as a phonendoscope. Soon thereafter, some of these devices were occasionally used (generally intermittently) during anesthesia and surgery. [4]
With time, stethoscopes secured to the chest were being employed more frequently during anesthesia for surgical procedures. There was even a description of a device designed so that both the surgeon and the anesthetist (often someone quite inexperienced) could simultaneously listen to heart and breath sounds during a procedure.[5]
One of the most impactful advocates for use of a precordial stethoscope during surgery, especially for children, was Dr. Robert Smith, (yes, the Robert Smith of the eponymous Smith award) who became the first Chief of Anesthesia at Boston Children’s Hospital in 1946 after he returned from military service in WWII.[6] By the 1970s, when we were anesthesia residents, routine use of a precordial stethoscope was mandatory during anesthesia, and all trainees were immediately fitted with a personalized, molded earpiece as a first initiation step to becoming an anesthesiologist. (And w still have our “earpieces”!)
The earpiece was a lifeline for both the patient and anesthesiologist. I (AJS) vividly remember being the sole anesthesia provider in a pediatric operating room, performing unassisted anesthetic inductions. With nobody able to secure an IV in a small pediatric vein, my halothane-oxygen mask induction would commence guided by listening to the heart tones via the earpiece tethering my ear to the precordial stethoscope. The brisk aurally detected cardiac cadence became muffled, signaling adequate depth of the anesthetic to allow laryngoscopy and endotracheal intubation without the assistance of muscle relaxants. I then tapped the ET tube in place, reduced the halothane concentration, allowing auscultation of the patient’s spontaneous respirations along with the more vigorous cardiac tones to guide the clinical care as I moved to the child’s hand to insert the IV. This completed the singlehanded anesthetic induction.
Use of the precordial stethoscope was at times problematic. Positioning it on the chest wasn’t possible if its presence was in the surgical field. Adequate positioning was difficult when the patient was prone. It was also quite annoying when the “surgical elbow” repeatedly knocked the stethoscope producing unhelpful extraneous sounds in the anesthesiologist’s ear. The solution to these annoyances was employing the esophageal stethoscope, which too was not without its risks.[7]
While historically, anesthesiologists would not dare to anesthetize patients without the use of a precordial or esophageal stethoscope, modern day practitioners because of the required use of pulse oximetry and capnography seem to have abandoned this clinical monitoring approach. A current observation (1995) of clinical practice performed during two weeks in three academic hospitals totalizing 57 operating rooms documented, “…an esophageal stethoscope was inserted in 68% of subjects, a precordial stethoscope was positioned in 16%, and an anesthetic stethoscope was absent in 16% of cases. Utilization (stethoscope connected to earpiece) ranged from a low of 11% of cases to a high of 45%, depending on the institution. Overall, providers were listening via an anesthetic stethoscope in only 28% of anesthetics…current anesthesia training may be fostering an environment where providers overlook a valuable minimally invasive, and cost-effective continuous monitor of patients…”. [8]
Perhaps an unintended consequence of the use of pulse oximetry and end-tidal capnography is abandoning the anesthesiologist’s stethoscopic connection to the patient. We are amazed that many trainees and practitioners arrive to the OR without any stethoscope! Thus, they are unable to auscultate breath sounds after inserting the ET tube or listen for wheezing that may be present when the oxygen saturation drops. A balanced perspective is necessary as use of pulse oximetry and end-tidal capnography is not the panacea. [9]
As written by Justin Skowno and Charlie Coté in the 7th edition of “A practice of Anesthesia for Infants and Children”, (CJ Coté, J Lerman and BJ Anderson, Elsevier 2025): “If both NIBP and the oximeter fail, but the child has strong heart sounds, a technical problem likely exists with the former two monitors. However, if the NIBP and pulse oximeter fail and the heart sounds are very weak, then cardiac output may be compromised, and attention should be immediately focused on resolving that problem rather than troubleshooting the monitors”. And, due to inherent delay of response of non invasive monitors, a decrease in heart or breathing sounds may herald a problem before any alarm sounds. But we must also acknowledge that it is difficult to interpret heart and breath sounds in the environment a modern operating room: monitors, voices, alarms or even music make the interpretation of acoustic signals more difficult. Although electronic, blue tooth enabled digital stethoscopes that can overcome these limits are currently commercially available, they have not gained widespread acceptance.[10]
On the other hand, many of our dentist anesthesiologist colleagues routinely use modern electronic precordial stethoscopes,[11] We will ask the PAAD’s executive council dentist anesthesiologists, Drs. Kyle Kramer and Lenny Naftalin, to write a future consumer report on these new precordials and how they are using them in their practice.
Do you use precordials in your practice? Have you ever seen one? Do you own one? Do you miss them? Send your thoughts and comments to Myron (myasterster@gmail.com) who will post in a Friday reader response.
References
1. Shephard DA: Harvey Cushing and anaesthesia. Can Anaesth Soc J 1965, 12(5):431–442.
2. Cushing H: Technical methods of performing certain cranial operations. Surg Gynec Obst 1908, 6(3):227–246.
3. Cushing H: Some principles of cerebral surgery. Jama 1909, 52(3):184–195.
4. Kirk R: On Auscultation of the Heart During Chloroform Narcosis. British medical journal 1896, 2(1876):1704–1706.
5. Kane E: Wearing of branching stethoscope by surgeons and anaesthetist during operation. Surg Gynecol Obstet 1924, 39:508–509.
6. Levin D, Rockoff MA: History of pediatric anesthesia. In: Smith’s Anesthesia for Infants and Children. Edited by Davis PJ, Cladis FP, 10th edn: Elsevier; 2022: 1462–1477.
7. Schwartz AJ, Downes JJ: Hazards of a simple monitoring device, the esophageal stethoscope. Anesthesiology 1977, 47(1):64–65.
8. Prielipp RC, Kelly JS, Roy RC: Use of esophageal or precordial stethoscopes by anesthesia providers: are we listening to our patients? Journal of clinical anesthesia 1995, 7(5):367–372.
9. Martinez MJ, Siegelman L: The new era of pretracheal/precordial stethoscopes. Pediatr Dent 1999, 21(7):455–457.
10. Kelmenson DA, Heath JK, Ball SA, Kaafarani HM, Baker EM, Yeh DD, Bittner EA, Eikermann M, Lee J: Prototype electronic stethoscope vs. conventional stethoscope for auscultation of heart sounds. J Med Eng Technol 2014, 38(6):307–310.
11. Kramer KJ, Herlich A: Did We Lose Something Along the Way? Anesth Prog 2022, 69(3):1–2.