Remembering the Classics: The “leak” test
Alan Jay Schwartz MD, MSEd and Justin L. Lockman MD, MSEd
The basis for understanding the issue:
When teaching residents and fellows I (AJS) always had the following discussion:
AJS to trainee: “Rumor has it you graduated from junior high school.”
Trainee response: “Of course”, was the reply with a quizzical look!
AJS: “So, you learned geometry in junior high school.”
Trainee: “Yes”, the quizzical look intensified!
AJS: “I’ll bet you didn’t know that geometry was your first pediatric anesthesia lesson. Geometry taught you that the cross-sectional area of a circle is defined as __?”
Trainee: “Pi r squared”
AJS: “Correct. What does that mean about the area of the circle when there is a reduction in r?”
Trainee (If insightful, responds): “A reduction in r results in an exponential reduction in the cross-sectional area of the circle.”
AJS: “By this logic, a reduction in the r of the trachea, a stack of circles, results in an exponential reduction in the cross-sectional area through which gas flows in and out of the airway. In pediatric care, this is critically important because in younger children the normal r is small to begin with. It obviously doesn’t take much reduction in the pediatric airway r to effect a clinically significant reduction in the air flow pathway and increase in the work of breathing. Grasping the clinical significance of this geometry is a key concept to seize. It is the basis upon which we devise our plans for safe pediatric airway management.” (See PS from Myron below).
The clinical application of airway geometry:
Because the pediatric airway is petite, anesthesiologists developed techniques attentive to safe airway management of this patient population. This entailed two clinical goals: (1) employ endotracheal tubes less likely to increase the child’s work of breathing and (2) minimize the risk of trauma/edema to the tracheal mucosa.
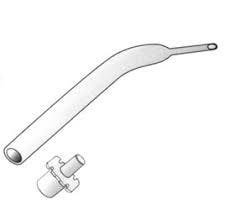
Focused on the goal to avoid increasing the work of breathing, Frank Cole, M.D. (1909-1982), devised, in 1945, an endotracheal tube specifically designed for pediatric patients.1
Figure: Drawing of the Cole tube http://ketteringsurgical.com/shop/surgical-instruments/catheters/cole-endotracheal-tube-21957.htm (accessed 06/24/2023).
The American Society of Anesthesiologists Wood Library Museum succinctly described the Cole tube: (https://www.woodlibrarymuseum.org/museum/cole-tracheal-tube/) (accessed 06/20/2023)
“Endotracheal tubes for infants need to be very narrow in diameter in order to fit through the larynx…Narrower tube diameters cause more resistance to the flow of air through the tube and can increase the work of breathing. Dr. Cole created his endotracheal tube so that it was narrowest only where it had to be: through or below the larynx.”
Focused on the goal to avoid trauma of the tracheal mucosa, Koka et al.2 in 1977 summarized their study of postintubation croup:
“Incidence of and contributory factors in postintubation laryngeal edema were determined in 7875 children under 17 years of age…With an overall incidence of 1 percent, children between ages 1 and 4 were most susceptible. Excessive size of the endotracheal tube was suspect in half of the cases. Other factors that increase trauma to the larynx while an endotracheal tube is in place showed significant correlation to the total incidence of postintubation laryngeal edema. No tracheostomies were required.”
It was intuitively obvious that less trauma produces less tracheal mucosal edema which better retains the trachea’s r when the endotracheal tube is removed. The education of anesthesiologists caring for pediatric patients advised against using cuffed endotracheal tubes because the presence of a cuff increases the external diameter of the endotracheal device and the inflated cuff increased pressure on the submucosa which resulted in edema.
So, in 1974, during my first clinical rotation learning pediatric anesthesia care, the teaching and clinical practice dictum when anesthetizing children <8 yo was never to use a cuffed endotracheal tube because the cuff increased the endotracheal tube’s external diameter resulting in a tube-trachea fit that was too snug and likely to produce mucosal edema. We either used an uncuffed or a Cole tube. This was especially true in those days when endotracheal tube cuffs were high-pressure, low volume devices prone to more tracheal trauma than the low-pressure compliant cuffs that subsequently became the standard. Once intubation was accomplished, we closed the pop off valve and listened with our stethoscopes over the larynx for the presence or absence of a leak. If no air leak was detected, the tube needed to be replaced with one of a smaller external diameter. We taught that post-extubation croup (stridor) was less likely when uncuffed tubes were employed.
While an uncuffed endotracheal tube had the advantage of a reduced external diameter and less potential for causing mucosal edema, the resulting unsealed airway had disadvantages including inability to perform adequate positive pressure ventilation, gastric aspiration risk and OR environmental pollution. Khine and colleagues3 demonstrated that these disadvantages could be overcome when a properly sized external diameter cuffed endotracheal tube was employed.
The possibility that endotracheal intubation induced tracheal mucosal trauma/swelling existed, posed a challenge whenever extubation was contemplated. How would a clinician know that the tracheal lumen would have sufficient r for adequate, non-turbulent, gas flow when the endotracheal tube was removed? Would a clinically significant reduction in airway cross sectional area be present? Would turbulent air flow/stridor occur? Would the work of breathing be increased, compromising the child’s ventilation and oxygenation capabilities? How would clinicians know that it was safe to remove the artificial airway?
The literature is replete with publications, some of which are cited here4-8 in addition to those cited in the recent PAAD consideration by Deshpande, Lockman and Yaster, “The ‘Leak test’ another ‘bubbameisa’ (old wives’ tale)?”, that have all investigated factors that substantiate or debunk the value of the leak test as a means to predict extubation safety. The multiple factors to be clinically considered are aptly summarized in many publications over the ensuing years from the 1970s to now.
Reviewing the many studies attempting to validate the leak test as a “fool proof” method to predict safety when extubating a pediatric patient reveals that it is not the single best assessment. The leak test is but one, important, clinical assessment predicting safety when contemplating endotracheal extubation of a child. The multiplicity of factors that may impact the tracheal r must be collectively reviewed to direct such clinical care.
PS from Myron: Two important teaching points (and my eternal thanks to my teachers Drs. Jeff Gross, Ted Smith, and Russ Raphaely): 1) One of the driving forces making the Cole tube so popular amongst pediatricians and anesthesiologists was that it made right main stem intubation almost impossible (One basically had to roto-rotor the tube past the vocal cords to accomplish a right main stem intubation). If you look carefully at the reproduced figure, you can see that only about 1-1.5 cm of endotracheal tube is below the taper/shelf. Thus, when placed properly, only 1-1.5 cm of tube was below the vocal cords and well above the right main stem bronchus. Remember, the trachea may only be 4 cm in length in the neonate. Unfortunately, this also meant that shelf of the Cole tube rested on the vocal cords which made edema and erosion very common realities. 2) Remember Poiseuille's Equation? Probably only in your worst nightmares. Poiseuille's Equation states that determinants of resistance to laminar flow are radius to the fourth power, length, and viscosity. Small decreases in diameter (radius) that can occur with tracheal or laryngeal edema when cuffed endotracheal tubes or when there was no leak with uncuffed tubes will cause tremendous increases in resistance even if the edema was minimal, particularly in our youngest and smallest patients. Turbulent flow which can easily occur with airway edema is even worse because resistance in turbulent flow is to the radius to the fifth power. Finally, the effects of viscosity explains why helium is often used in patients with severe airway resistance and croup. Hope this helps some of you with your Board exams!
References
1. Cole F. An endotracheal tube for babies. Anesthesiology. Nov 1945;6:627.
2. Koka BV, Jeon IS, Andre JM, MacKay I, Smith RM. Postintubation croup in children. AnesthAnalg. 1977 1977;56(4):501-505. Not in File.
3. Khine HH, Corddry DH, Kettrick RG, et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997 1997;86(3):627-31; discussion 27A. Not in File.
4. Finholt DA, Henry DB, Raphaely RC. Factors affecting leak around tracheal tubes in children. CanAnaesthSocJ. 7/1985 1985;32(4):326-329. Not in File.
5. Adderley RJ, Mullins GC. When to extubate the croup patient: the "leak" test. Canadian journal of anaesthesia = Journal canadien d'anesthesie. May 1987;34(3 ( Pt 1)):304-6. doi:10.1007/bf03015171
6. Schwartz RE, Stayer SA, Pasquariello CA. Tracheal tube leak test--is there inter-observer agreement? Canadian journal of anaesthesia = Journal canadien d'anesthesie. Nov 1993;40(11):1049-52. doi:10.1007/bf03009476
7. James I. Cuffed tubes in children. Paediatric anaesthesia. May 2001;11(3):259-63. doi:10.1046/j.1460-9592.2001.00675.x
8. Argalious MY. The cuff leak test: does it "leak" any information? Respiratory care. Dec 2012;57(12):2136-7. doi:10.4187/respcare.02193