Opioids are the fundamental building blocks of anesthesia and pain management. However, they are “dirty” drugs with a host of effects beyond analgesia including nausea/vomiting, bowel dysmotility, sedation, miosis, and the development of tolerance and dependence. Obviously, the most important and catastrophic effect is respiratory depression which can lead to cardiovascular collapse and death. What if there was an opioid that didn’t produce respiratory depression (or much more limited respiratory depression) and/or the development of tolerance? Ahhh, the quest for the holy grail…
Almost all of the opioids like fentanyl and morphine that we use in our daily practice work by specifically binding to the mu-opioid receptor, a member of the G-Protein Coupled Receptors (GPCRs) family. Methadone is an exception in that it binds not only to the mu-receptor but also binds (as an antagonist) to an NMDA receptor. Once the agonist, say morphine or fentanyl binds to the mu-agonist receptor a cascade of intracellular events occur that open and close potassium and calcium channels, activate cyclic AMP and phosphorylate proteins with extensive downstream effects. About 20 years ago Dr. Laura Bohn and her colleagues discovered that beta-arrestins bind to some of these activated phosphorylated proteins and essentially “turn off” the activated G-protein activated recetor.1 Bohn speculated that if an opioid was developed that limited recruitment of the beta arrestins, there would be significantly less respiratory depression and tolerance. Based on her work, new designer opioids that are “biased” away from beta arrestin recruitment were developed or are in devolpment.
Oliceridine (Olinvyk™ Trevena Inc, Chesterbrook, Pennsylvania, USA) is the first of these new designer biased ligands and was recently approved by the FDA. Theoretically and in adult human trials, oliceridine has a lower probability of producing respiratory depression than classic mu opioids like morphine.2, 3
I don’t know if any of you have used this drug yet in pediatric practice nor do I know if anyone is studying it. Indeed, in a quick look up, I dont think that Trevena Inc has sold much of it to anyone. Let me know if you have experience with oliceridine and I will post your response in the Friday Reader Response PAAD. Thus, today’s PAAD is more of a future BOLO or “Be On the Look Out” for than an article that will affect your practice today. There are a lot of other very interesting study design issues in today’s PAAD that I will try to highlight. But first, a word from Monty Python and the Quest for the Holy Grail….Myron Yaster MD
Original article
Pieter Simons, Rutger van der Schrier, Maarten van Lemmen, Simone Jansen, Kiki W K Kuijpers, Monique van Velzen, Elise Sarton, Todd Nicklas, Cathy Michalsky, Mark A Demitrack, Michael Fossler, Erik Olofsen, Marieke Niesters, Albert Dahan. Respiratory Effects of Biased Ligand Oliceridine in Older Volunteers: A Pharmacokinetic-Pharmacodynamic Comparison with Morphine. Anesthesiology 2023 Mar 1;138(3):249-263 PMID: 36538359
Today’s PAAD comes from the laboratory of Dr. Albert Dahan from the Leiden University Medical Center in the Netherlands.4 He and his team are the world leaders in anesthetic drug pharmacokinetics and dynamics. Indeed, anytime I see an article from this group I make it a point to read it and attempt to understand the methodology. The authors “hypothesized that oliceridine and morphine differ in their pharmacodynamic behavior, measured as effect on ventilation at an extrapolated end-tidal Pco2 of 55 mmHg”.4 The study, a randomized controlled, double blinded prospective trial, used a population consisting of healthy volunteers 55 years of age or older and with a body mass index in the range 19 to 35 kg/m2 (inclusive). A third of the patients had BMIs between 30-35 kg/m2.
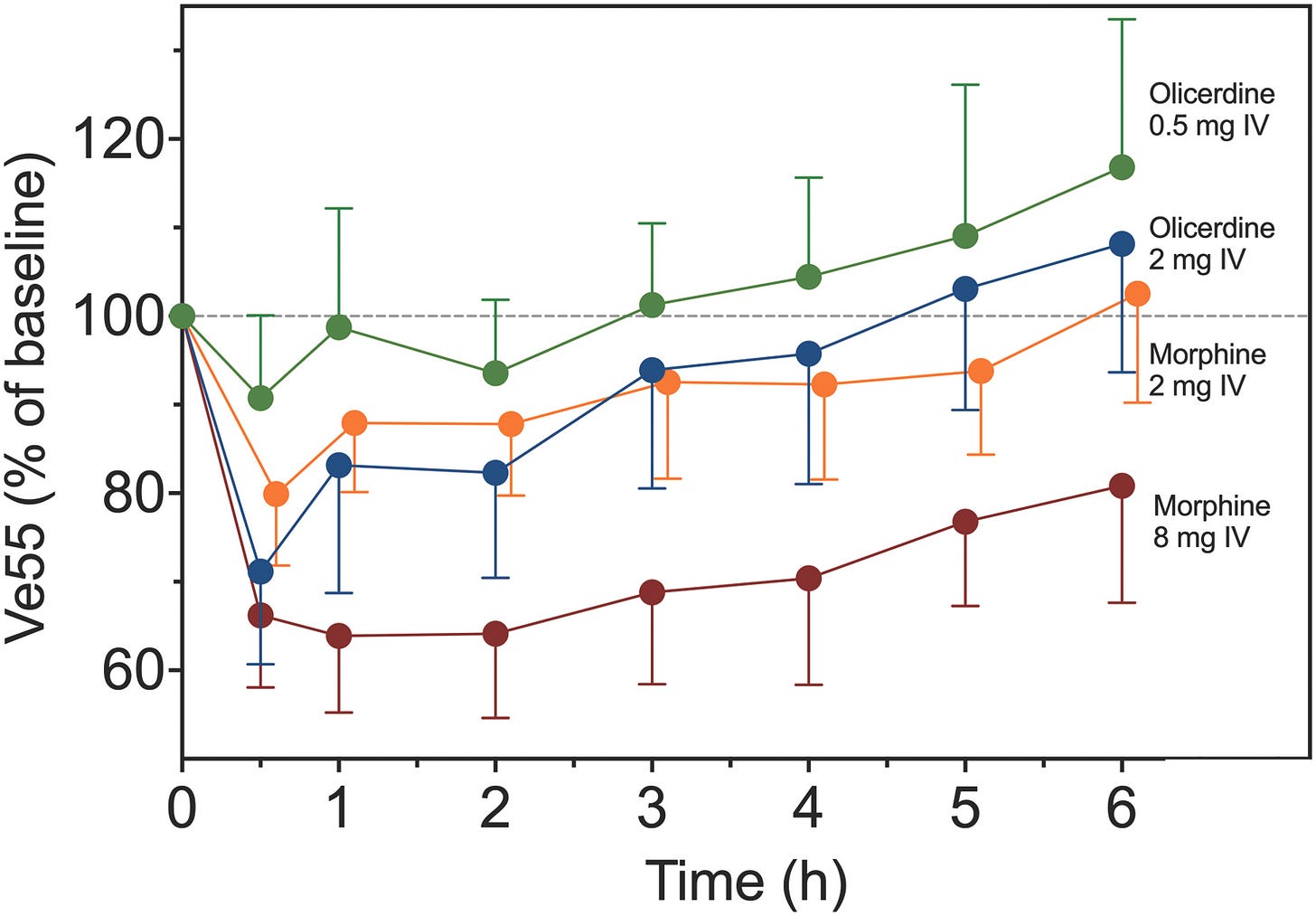
Oliceridine is approximately 4 times more potent than morphine. At different times over the course of the study, patients received either 0.5mg (low) or 2 mg (high) oliceridine or 2 mg (low) or 8 mg (high) morphine by a bolus given over 1 minute. The respiratory effects of the drugs were measured using a modified classic rebreathing method. This technique uses a face mask attached to pneumotachograph to measure ventilation breath by breath and a capnomometer to measure inspired and expired CO2. I used this technique 30 years ago in a study looking at the respiratory effects of spinally administered morphine in children. 5 It is a simple and elegant technique that is easily used in pediatric studies.
“The pharmacokinetics and pharmacodynamics of oliceridine and morphine were analyzed with NONMEM VII (Icon Plc., USA), a software package for nonlinear mixed-effects modeling, using a population approach”.4 The authors used allometric scaling to fit their pharmacokinetic findings to their models. Many of you may be unfamiliar with allosteric scaling and should be. In our every day pediatric anesthesia practice we use mg/kg in making our drug dose calculations. This often works but fails when patients have large BMIs. How do you dose those patients? Lean body weight? Actual weight? Ask the pharmacist? Interestingly, the best pharmacologic method used in studies like in today’s PAAD is to use allometric scaling conversion.6 A discussion of how to make and use this scaling conversion is beyond the scope of today’s PAAD but I think it may be worth a future PAAD or SPA PEDx talk. I’ll try to find a good review and post in the near future. Finally, the authors also looked at morphine and morphine- metabolites and genotyped all of the study subjects for CYP2D6.
What did they find? “(1) there was a 30% difference in respiratory potency between oliceridine and morphine with a 50% reduction of V̇E55 (C50) observed at 29.9 ± 3.5 ng/ml oliceridine and 21.1 ± 4.6 ng/ml morphine; (2) oliceridine had a 5-times faster onset and offset of respiratory effect than morphine (blood-effect-site equilibration half-life, t½ke0, 44 ± 6 min for oliceridine versus 214 ± 27 min for morphine); and (3) oliceridine metabolism was dependent on the CYP2D6 enzyme genotype. Simulations revealed that about 40% less oliceridine is needed to achieve the same level of respiratory depression in poor metabolizers compared with normal metabolizers over 24 h.”(figure)4
OK, in English this time….Oliceridine has a faster onset and offset of respiratory than morphine AND produces 30% less respiratory depression than morphine. Not yet the holy grail but biased ligands may be how we use opioids in the future.
References
1. Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 12/7/2000 2000;408(6813):720-723. In File.
2. Dahan A, van Dam CJ, Niesters M, et al. Benefit and Risk Evaluation of Biased μ-Receptor Agonist Oliceridine versus Morphine. Anesthesiology. Sep 2020;133(3):559-568. doi:10.1097/aln.0000000000003441
3. Bergese S, Berkowitz R, Rider P, et al. Low Incidence of Postoperative Respiratory Depression with Oliceridine Compared to Morphine: A Retrospective Chart Analysis. Pain research & management : the journal of the Canadian Pain Society = journal de la societe canadienne pour le traitement de la douleur. 2020;2020:7492865. doi:10.1155/2020/7492865
4. Simons P, van der Schrier R, van Lemmen M, et al. Respiratory Effects of Biased Ligand Oliceridine in Older Volunteers: A Pharmacokinetic-Pharmacodynamic Comparison with Morphine. Anesthesiology. Mar 1 2023;138(3):249-263. doi:10.1097/aln.0000000000004473
5. Nichols DG, Yaster M, Lynn AM, et al. Disposition and respiratory effects of intrathecal morphine in children. Anesthesiology. 1993 1993;79(4):733-8; discussion 25A. Not in File.
6. Holford NH. A size standard for pharmacokinetics. Clinical pharmacokinetics. May 1996;30(5):329-32. doi:10.2165/00003088-199630050-00001