About 30 years ago, I was called in the middle of the night by the Hopkins PICU attending and my best friend, Dr. David Nichols, to “volunteer” to transport a child with an obstructed VP shunt from the Eastern shore of Maryland to Hopkins. I wasn’t on call but the PICU, the ORs and the transport system were overwhelmed and I was the closest attending to the hospital. I must admit that I was annoyed and sure that on arrival to the local hospital the child would be awake, playful and probably ask “where’s my mommy?” After a brief helicopter flight (and I really hated them!), I found the child in extremis…bradycardic, agonal breathing, and with dilated pupils. The shunt bulb could not be compressed. My diagnosis? Elevated ICP and a shunt obstruction. I performed a rapid sequence intubation and after taping the tube asked my transport team to hyperventilate. No improvement. I prepped the skin and using a butterfly needle tapped the shunt bulb and no fluid…I asked for a spinal tray, reprepped the skin and aiming at the bridge of the nose, inserted a 22 gauge spinal needle down the shunt’s tract into the lateral ventricle (a lumbar puncture to remove CSF is contraindicated in this situation because it can cause herniation). When I removed the needle’s stylette, CSF shot out and I think hit the ceiling. I then attached a T connector and syringe and withdrew more CSF for culture and sensitivity and started antibiotics. The child’s heart rate increased to normal and pupils became reactive. We rapidly returned to Hopkins and went directly to the OR. I’ll never forget what David told me when the dust settled: “that the kid was lucky I was the transport doctor…few others would have dared to do what I did”. We ended up putting how to do this into our pediatric handbook the Golden Hour: the handbook of Advanced Pediatric Life Support .
We all anesthetize children with hydrocephalus, often repeatedly. This is a very good review article with fantastic illustrations. I am copying one of the figures for you. I’d also like to thank Dr. Alan Klein, a frequent reader of the PAAD, who alerted me to this article when it was e-published before print. Myron Yaster MD
Review article
Whitehead WE, Weiner HL. Infantile and Childhood Hydrocephalus. N Engl J Med. 2022 Dec 1;387(22):2067-2073. doi: 10.1056/NEJMra2116504. PMID: 36449422
“Approximately 400,000 cases of infantile and childhood hydrocephalus occur worldwide annually. Various impediments, congenital or acquired, can derange the CSF circulation and result in hydrocephalus. Common congenital causes are myelomeningocele, aqueductal stenosis, and posterior fossa malformations (particularly Chiari and Dandy–Walker malformations). Acquired disorders that commonly cause hydrocephalus are tumors, hemorrhages, and infections. The most common cause of hydrocephalus globally in infants and children is infection. Hydrocephalus often follows neonatal sepsis and can be caused by a variety of pathogens. In the United States, the most common cause of hydrocephalus in the pediatric population is intraventricular hemorrhage associated with prematurity.”1, 2
“Neonates rarely present in distress from hydrocephalus because their open fontanelles and skull sutures decompress the additional volume of the expanded ventricles. The clinical features of hydrocephalus in neonates include a head circumference that is larger than expected, a bulging fontanelle, and widely separated cranial sutures. Progression of ventricular enlargement may lead to a downward deviated gaze, irritability, lethargy, episodes of apnea and bradycardia, and developmental regression.”1
“In older children, whose fontanelles are already closed and can no longer decompress the intracranial contents, hydrocephalus has more acute manifestations, with headache, nausea, vomiting, double vision, lethargy, and occasionally, seizures. In advanced cases, children have bradycardia, papilledema, and sixth-nerve palsies. The history may uncover regression of developmental milestones and difficulties with school.”1
“The CSF shunt is the mainstay of treatment for hydrocephalus. Because shunts have high failure rates, alternative treatments have been proposed that may improve quality of life.”1 How high is the failure rate? A neurosurgeon friend that I knew well called them “annuities” because “approximately 30 to 40% of shunts placed in infants fail in the first year, 40 to 50% fail by 2 years, and 80 to 90% fail by 10 years. The average patient undergoes 2.5 shunt revisions during childhood, with 5% requiring 10 or more revisions.”1, 3 Obstruction and infection are the most causes of failure.
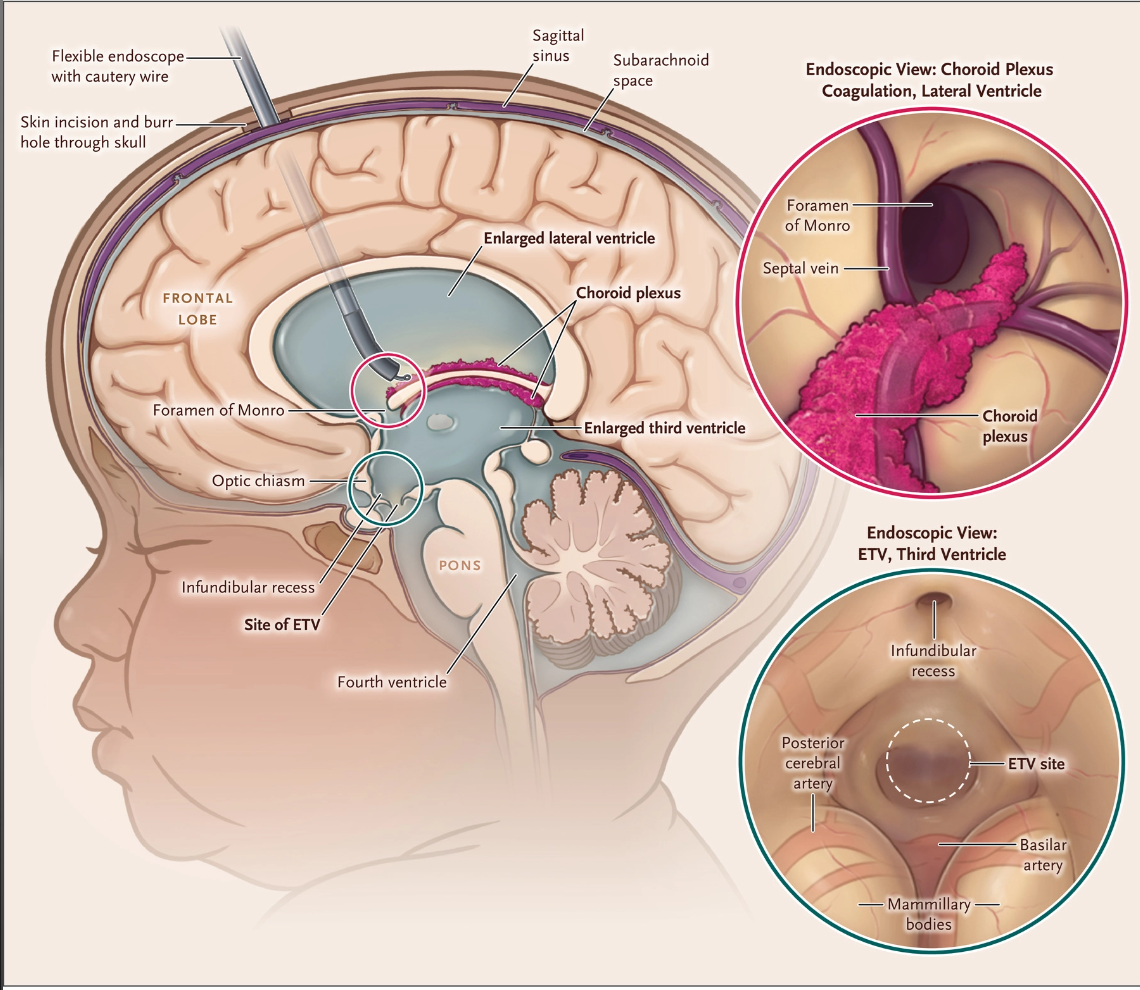
An alternative to CSF shunting, first developed for resource poor countries, is endoscopic third ventriculostomy (ETV).4, 5 In this procedure, an endoscope is passed through a frontal scalp incision into the third ventricle, and an opening is made in the ventricular floor which allows CSF to flow between the 3d ventricle and subarachnoid space just below it. 5, 6 “To improve the chances of successful ETV in infants, choroid plexus cauterization to limit CSF production can be performed as an adjunct procedure at the same time as ETV.1, 5, 6 “A North American randomized trial comparing the combined surgery with shunting alone is under way (ClinicalTrials.gov number, NCT04177914).”1
There are some fantastic images/illustrations in this paper that many of you who teach may find very useful. We are attaching this drawing for all of you to see.
“Figure 2. Endoscopic Choroid Plexus Cauterization Performed with the Use of a Flexible Endoscope with a Working Channel after ETV.
A flexible endoscope with a working channel is passed through the right frontal cortical mantle into the frontal horn of the lateral ventricle. After the ETV has been completed, a cautery wire is used to coagulate the choroid plexus in the body of the lateral ventricle. Coagulation of the choroid plexus continues to the temporal horn, and then the contralateral choroid plexus is coagulated to the temporal horns through a septostomy.”1
The article does not discuss the anesthetic management of shunt or endoscopic third ventriculostomy with or without choroid plexus cauterization nor the management of these patients in the presence of increased intracranial pressure. Do you have any pearls of wisdom you’d like to share with the PAAD readership? Drop Myron an email and we’ll post in a reader’s response.
References
1. Whitehead WE, Weiner HL. Infantile and Childhood Hydrocephalus. The New England journal of medicine. Dec 1 2022;387(22):2067-2073. doi:10.1056/NEJMra2116504
2. Dewan MC, Rattani A, Mekary R, et al. Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. Journal of neurosurgery. Apr 1 2018:1-15. doi:10.3171/2017.10.Jns17439
3. Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ. Revision rate of pediatric ventriculoperitoneal shunts after 15 years. Journal of neurosurgery Pediatrics. Jan 2013;11(1):15-9. doi:10.3171/2012.9.Peds1298
4. Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E, et al. Endoscopic Treatment versus Shunting for Infant Hydrocephalus in Uganda. The New England journal of medicine. Dec 21 2017;377(25):2456-2464. doi:10.1056/NEJMoa1707568
5. Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. Journal of neurosurgery. Dec 2005;103(6 Suppl):475-81. doi:10.3171/ped.2005.103.6.0475
6. Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S. Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. The Journal of pediatrics. Aug 2009;155(2):254-9.e1. doi:10.1016/j.jpeds.2009.02.048