If You Are Looking To Improve Cardiac Output After Tetralogy of Fallot Repair You Might Need to Consider A Drug Other Than Milrinone
Susan Nicolson, Lindsey Loveland Baptist, Viviane Nasr, James DiNardo
“Tetralogy of Fallot (T0F) is the most common cyanotic congenital heart disease (CHD), and children typically undergo surgical repair before 1 year of age. The corrective operation typically includes surgical relief of right ventricular (RV) outflow tract obstruction (via transannular myectomy, infundibulotomy, or transannular patch), closure of the ventricular septal defect (VSD), and restriction or closure of the interatrial shunt. Outcomes following repair are excellent, and perioperative mortality is rare.”1 In today’s PAAD, our cardiac anesthesia team reviews an article by Saengsin et al. looking at the association between administration of milrinone and volume administration during the first 72 hours after surgery. Myron Yaster MD
Original article
Saengsin K, Sperotto F, Lu M, Garcia Mancebo J, Sacco E, Godsay M, DiNardo JA, Kheir JN. Administration of Milrinone Following Tetralogy of Fallot Repair Increases Postoperative Volume Administration Without Improving Cardiac Output. Anesth Analg. 2023 Nov 1;137(5):1056-1065. doi: 10.1213/ANE.0000000000006662. Epub 2023 Oct 20. PMID: 37733944.
Milrinone, a phosphodiesterase inhibitor, inhibits the intracellular breakdown of 3,5’-cyclic adenosine monophosphate (cAMP), enhancing intracellular calcium transport. This results in direct, dose-dependent vasodilatory effects on systemic and pulmonary veins, and on the peripheral and cerebral vasculature.2,3 Vasodilation, along with inotropic effect is thought to be responsible for clinical improvement in congestive pathologies.1
Use of milrinone following congenital heart surgery was examined in the PRophylactic Intravenous use of Milrinone After Cardiac OpeRation in Pediatrics (PRIMACORP) study.4 This randomized controlled trial found that administration of high dose milrinone (75 ug/kg bolus followed by 0.75 ug/kg/min infusion) reduced the incidence of low cardiac output syndrome (LCOS) after pediatric cardiac surgery, including repair of Tetralogy of Fallot (ToF). Many clinicians use milrinone to mitigate or treat LCOS after pediatric open heart surgery.5
The authors sought to explore the association between use of perioperative (intraoperative or within the first 72 postoperative hours) milrinone and volume administration during the 1st 72 hours after repair of ToF. Volume administration included blood products and 5% albumin. Volume associated with medications, flushes, infusions and nutritive fluids (parental nutrition, intralipids or dextrose containing IV fluids) were excluded, as these fluids were not administered as volume replacement. They hypothesized that in children with restrictive ventricular physiology use of milrinone would be associated with increased volume administration, increased heart rate, decreased blood pressure without significant improvement in surrogates for cardiac output.
Of the 351 patients who had primary surgical repair of ToF at Boston Children’s Hospital between January 2011 and December 2020, only 134 received perioperative milrinone. Its use was directed by the practitioners caring for the child and was not protocolized, thus the timing and dosage was variable and not detailed in the paper. Subjects with and without milrinone were matched in a 1:1 ratio according to propensity score by optimal method. A total of 212 children (106 received milrinone, 106 did not) were matched.
Milrinone patients were younger, had lower weight, had smaller pulmonary and tricuspid valves and main pulmonary artery Z-scores and more commonly received a transannular patch (performed in the setting of a more restrictive native RV outflow tract).
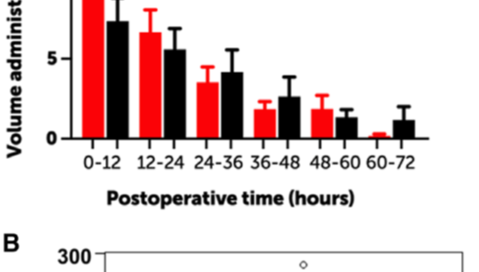
Milrinone-treated patients received postoperative volume more frequently than patients who had not received milrinone (66% vs 52%). The total volume administered during the 1st 72 postoperative hours in the milrinone-treated patients was associated with the total dose of postoperative milrinone. Linear regression modeling was used to explore the relationship between total dose of milrinone and total volume administered. For every 1000 ug/kg of milrinone (equivalent to about 0.25 ug/kg/min for 72 hours), 11.0 +/- 5.2 mlkg of additional volume was administered (Figure)
Compared to the non- milrinone exposed cohort, the milrinone cohort was associated with a higher heart rate throughout the 1st 72 postoperative hours, had a slower resolution of tachycardiac during the 1st 12 hours and a clinically insignificant lower blood pressure on admission to the ICU. Lower blood pressures combined with a higher heart rate and increased likelihood of volume administration may be explained by the vasodilatory effect of milrinone with a compensatory chronotropic response.
No significant differences were observed between groups in values of markers of oxygenation or perfusion, including arterial or venous oxyhemoglobin saturation arteriovenous oxyhemogobin (AVO2) difference, or serum lactate.
“In contrast to congestive heart failure, the clinical phenotype after ToF repair is restrictive ventricular physiology. The hypertrophied RV, ventriculotomy, infundibular and VSD patch and myocardial edema all combine to diminish RV capacitance. In this setting LCOS is a manifestation of diminished RVEDV (ie. preload) as a proximate cause for diminished stroke volume and cardiac output. In the absence of atrial shunting, maximizing cardiac output requires filling the “stiff” right ventricle. Interventions that venodilate, like milrinone, increase systemic venous capacitance, diminishing RVEDV, stroke volume and cardiac output.”1
This study has several limitations including its retrospective nature over a span of a decade, selection bias and lack of a protocol for dosing and timing of administration of milrinone and administration of postoperative volume.
There is a need to prospectively evaluate the association between the use of milrinone and postoperative hemodynamics, including cardiac output in children with restrictive cardiac physiologies.
Has today’s PAAD changed your understanding of the use of milrinone in ToF or in other conditions with restrictive physiology? Send your thoughts and comments to Myron who will post in a Friday Reader response.
References
1. Saengsin K, Sperotto F, Lu M, Garcia Mancebo J, Sacco E, Godsay M, DiNardo JA, Kheir JN: Administration of Milrinone Following Tetralogy of Fallot Repair Increases Postoperative Volume Administration Without Improving Cardiac Output. Anesth Analg 2023; 137: 1056-1065
2. Rieg AD, Suleiman S, Perez-Bouza A, Braunschweig T, Spillner JW, Schröder T, Verjans E, Schälte G, Rossaint R, Uhlig S, Martin C: Milrinone relaxes pulmonary veins in guinea pigs and humans. PLoS One 2014; 9: e87685
3. Abulhasan YB, Ortiz Jimenez J, Teitelbaum J, Simoneau G, Angle MR: Milrinone for refractory cerebral vasospasm with delayed cerebral ischemia. J Neurosurg 2020; 134: 971-982
4. Hoffman TM, Wernovsky G, Atz AM, Kulik TJ, Nelson DP, Chang AC, Bailey JM, Akbary A, Kocsis JF, Kaczmarek R, Spray TL, Wessel DL: Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation 2003; 107: 996-1002
5. Burkhardt BE, Rücker G, Stiller B: Prophylactic milrinone for the prevention of low cardiac output syndrome and mortality in children undergoing surgery for congenital heart disease. Cochrane Database Syst Rev 2015: Cd009515
Figure. Volume Administration
A. Volume administration over the 1st 72 postoperative hours by 12 hour intervals
B. Total volume administered in the 1st 72 postoperative hours increased with total milrinone dose during the same time frame. The flexible fit between these 2 variables estimated by generalized additive modeling (blue line) suggests the that relationship is linear. The red line depict the results of a linear regression model.
Figure 2
Intravascular volume, depicted by the column, can be subdivided into unstressed volume (blue) and stressed volume (red); only stressed volume generates the pressure head for ventricular filling. Unstressed volume represents the volume that must be filled before stressed volume is achieved. In the healthy state (A), a low-stressed volume (and atrial pressure) is required to achieve a normal EDV because ventricular compliance is normal. In restrictive ventricular physiology (B), this same low central venous pressure results in a lower EDV and stroke volume; such patients require a higher atrial pressure to maintain normal preload. In this circulation, vasodilators increase venous capacitance, reducing the fraction of the circulation devoted to stressed volume (C), lowering atrial pressure, EDV and stroke volume; this physiology, particularly when combined with arteriolar dilation, can result in hypotension. The recruitment of stroke work in this situation can be achieved through the administration of volume (D) or by the reduction in venodilated state (eg, reducing venodilation or inducing venoconstriction). In contrast, venoconstrictors decrease venous capacitance, raise stressed volume, centralize blood volume and increase stroke volume for a given intravascular volume (E). EDV indicates end-diastolic volume.