From Bench To Bedside – Reducing The Heart Transplant Wait List Itaconate and Organ Preservation; You Just Can’t Escape the TCA Cycle
James DiNardo MD, Viviane Nasr MS, Susan Nicolson MS, Lindsey Loveland-Baptist MD
Every once in awhile when reading a journal article or a PAAD by our contributors, I have an “Ah ha” moment. Today’s PAAD by our pediatric cardiac anesthesia editors was just such a moment. I was blown away by its implications. PLEASE read this to the end and DON’T abandon ship or convulse when you see the figure of the TCA cycle. It will be well worth your time!
The TCA cycle? Yes the TCA cycle. The tricarboxylic acid (TCA) cycle, also known as the Krebs or citric acid cycle. Oh, the nightmares of having to memorize this for college chemistry and the first year biochemistry class in medical school. I remember the nightmares of failing so clearly! I’m sure most of you felt the same as I did. Of what possible use was the Krebs cycle for a budding clinician? To clear the cobwebs remember that the Krebs cycle is the main source of energy for cells and an important part of aerobic respiration. The cycle harnesses the available chemical energy of acetyl coenzyme A (acetyl CoA) into the reducing power of nicotinamide adenine dinucleotide (NADH). It turns out it is also important in inflammation and immunity. Who knew? Myron Yaster MD
Original article
Lei I, Huang W, Noly PE, Naik S, Ghali M, Liu L, Pagani FD, El Ela AA, Pober JS, Pitt B, Platt JL, Cascalho M, Wang Z, Chen YE, Montensen TM, Tang PC. Metabolic Re-programming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci Transl Med. 2023 Feb 8;15(682):eade3782. doi: 10.1126/scitranslmed.ade3782. Epub 2023 Feb 8. PMID: 36753565
Orthotopic heart transplantation remains, 50 years after the first successful transplant, the most effective treatment for end-stage heart failure. Unfortunately, a shortage in donor hearts deprives many eligible patients from receiving this life saving therapy. Less than 50% of potential donors become actual heart donors. Furthermore, prolonged ischemic time is a risk factor for primary graft dysfunction (PGD), Therefore, a 4-hour limit on ischemia time following use of the traditional technique of diastolic arrest with cardioplegia followed immediately by cold storage is generally adhered to. To keep flight time less than two hours, the donor heart is generally procured within a radius of five hundred miles of the recipient which restricts the distribution of potential donor hearts from distant locations due to prolonged transport times.
Improving heart preservation techniques then is a key to increasing the donor pool. One approach is the use of the Organ Care System (OCS). OCS heart is a portable extracorporeal heart perfusion and monitoring system which allows for the resuscitation, preservation, and assessment of donor's hearts in a near-physiologic, normothermic, and beating state. It allows continuous monitoring of aortic pressure, lactate level, and coronary blood flow.1 OCS potentially allows prolongation of transport times beyond 4 hours by keeping the heart perfused close to a normal physiological state. Unfortunately, this system does not eliminate the occurrence of PGD which has led others to pursue alternative methods of improving preservation.2
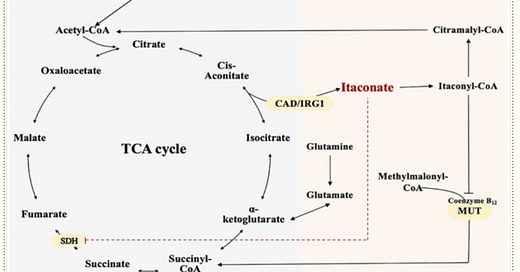
That brings us to today’s article.2 Macrophages activated by numerous inflammatory mediators such as lipopolysaccharide increase the expression of immune response gene 1 (IRG1), also known as aconitate decarboxylase 1 (ACOD1). This increases glycolytic flux and pushes the tricarboxylic acid (TCA) cycle toward production of itaconate by decarboxylating cis-aconitate. High production of itaconate inhibits succinate dehydrogenase, blocking succinate-mediated inflammatory processes and inducing the anti-inflammatory proteins nuclear factor erythroid 2-related factor 2 (NRF2) and ATF3.3, 4 Itaconate is a key autocrine regulatory component involved in the development and progression of inflammation and immunity. It is a link between immunity, metabolism, and inflammation, and as such has multiple immunoregulatory and antioxidative effects (figure)5
In today’s manuscript the authors employed murine, porcine, and human heart models to demonstrate that the cardioprotective effects of itaconate can improve donor heart ischemic tolerance and function. The authors administered valproic acid (VPA), an inhibitor of histone deacetylase (HDAC). HDACs are enzymes that remove acetyl groups from lysine residues in the NH2 terminal tails of DNA histones, resulting in a more closed chromatin structure and repression of gene expression. HDAC inhibitors can therefore potentially be used to mitigate inflammation by promoting the hyperacetylation states of histones. In this model inhibition of HDAC was shown to induce up-regulation of IRG1.
A murine heart tissue, a murine ex vivo perfusion model, and a murine heterotopic heart transplant model demonstrated the cardioprotective effects, both metabolic and functional, of VPA treatment. A porcine heart tissue and a porcine ex vivo perfusion model also demonstrated the cardioprotective effects, both metabolic and functional, of VPA treatment. Human heart tissue analysis was conducted using human donor hearts from deceased brain death donors procured as per standard clinical protocols from the Gift of Life Michigan organ procurement facility. Myocardial biopsies of the left ventricle immediately after cardioplegic arrest (0 hours) and after 8 hours of cold storage in a cooler containing ice were studied. These biopsies revealed VPA treatment reduced succinate accumulation in human donor hearts during cold preservation and was associated with increased itaconate availability. As anticipated, increased IRG1 expression contributed to this improved cardiac preservation quality and cardiomyocyte survival after VPA treatment.
Why is this important you might well ask? It is important because as clinicians we can never lose sight of the incredible creativity and ingenuity of the basic science and translational researchers. Nor should we ever lose the ability to critically read a basic science or translational research paper. This type of sophisticated, painstaking research lays the groundwork for development of the new medications and methods that allow us as clinicians to improve the lives of others.
References
1. Alomari M, Garg P, Yazji JH, et al. Is the Organ Care System (OCS) Still the First Choice With Emerging New Strategies for Donation After Circulatory Death (DCD) in Heart Transplant? Cureus. Jun 2022;14(6):e26281. doi:10.7759/cureus.26281
2. Lei I, Huang W, Noly PE, et al. Metabolic reprogramming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci Transl Med. Feb 8 2023;15(682):eade3782. doi:10.1126/scitranslmed.ade3782
3. O'Neill LAJ, Artyomov MN. Itaconate: the poster child of metabolic reprogramming in macrophage function. Nat Rev Immunol. May 2019;19(5):273-281. doi:10.1038/s41577-019-0128-5
4. Li R, Zhang P, Wang Y, Tao K. Itaconate: A Metabolite Regulates Inflammation Response and Oxidative Stress. Oxid Med Cell Longev. 2020;2020:5404780. doi:10.1155/2020/5404780
5. Lin J, Ren J, Gao DS, Dai Y, Yu L. The Emerging Application of Itaconate: Promising Molecular Targets and Therapeutic Opportunities. Front Chem. 2021;9:669308. doi:10.3389/fchem.2021.669308