Challenge of Neonatal Anesthesia: Which Optimal EEG Target?
Myron Yaster MD and Jerry Chao MD
Today’s PAAD by Corlette et al1 and its accompanying editorial by Constant2 is a review of the use of EEG to define the depth of anesthesia in very young infants who have limited brain EEG activity in the first place. Indeed, I have this indelible memory from my pediatric residency when I was told by a senior resident “that there was little to learn from a neonate’s EEG because their brains are so immature that the wave patterns and amplitudes are the same as a shaken Jello mold!” Obviously, this was never true and we’ve come a long way in understanding brain development and neonatal EEGs. Nevertheless, the underlying reality remains: the newborn has very different EEG waves, patterns, and amplitudes compared to adults and older children. Thus, in today’s PAAD, we’ll answer the question: “can we use the EEG intra-operatively to guide anesthetic delivery in the newborn?” Myron Yaster MD
Editorial
Constant I. Challenge of Neonatal Anesthesia: Which Optimal EEG Target? Anesthesiology. 2024 Oct 1;141(4):632-634. doi: 10.1097/ALN.0000000000005157. PMID: 39254535.
Original article
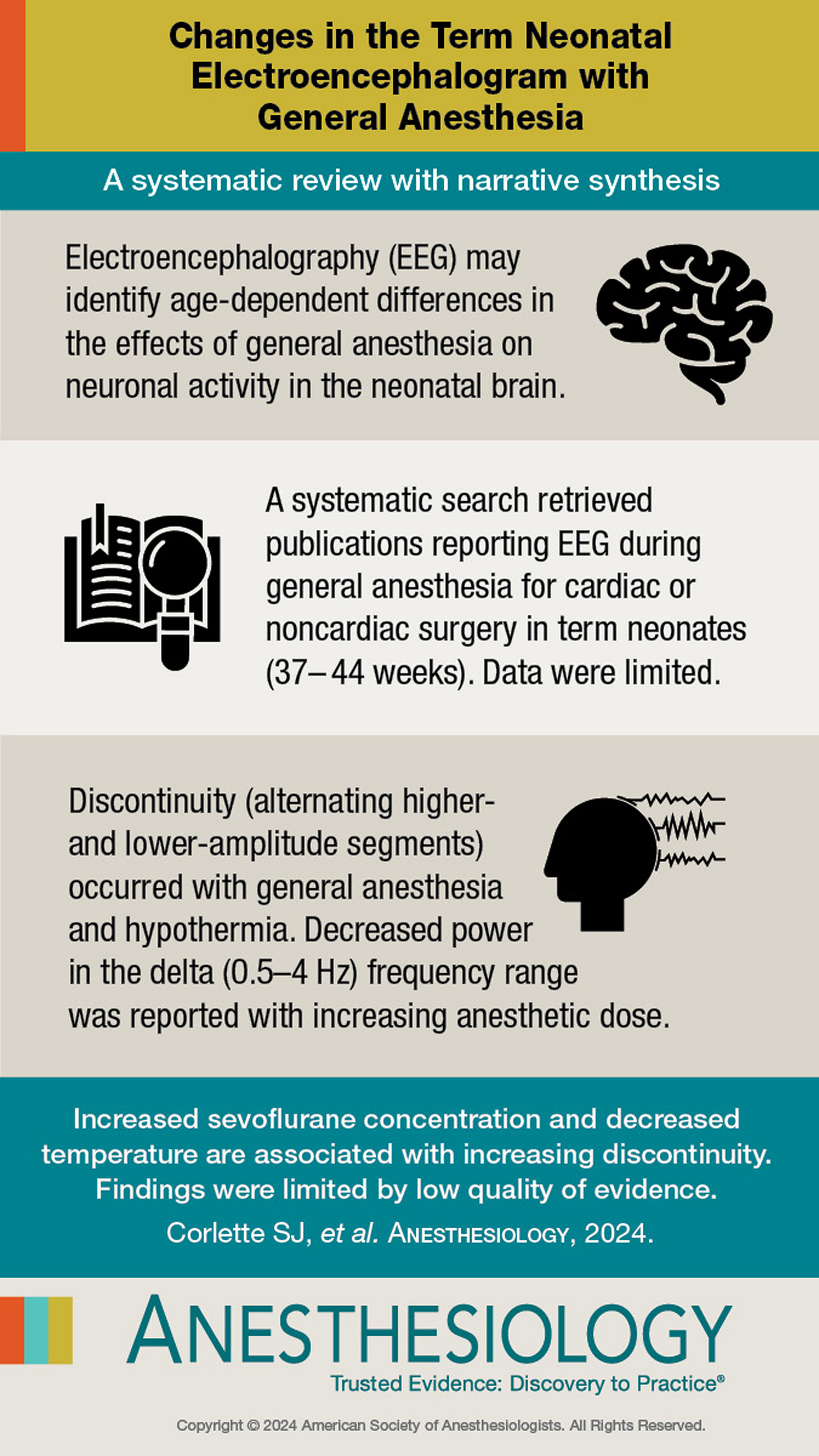
Corlette SJ, Walker SM, Cornelissen L, Brasher C, Bower J, Davidson AJ. Changes in the Term Neonatal Electroencephalogram with General Anesthesia: A Systematic Review with Narrative Synthesis. Anesthesiology. 2024 Oct 1;141(4):670-680. doi: 10.1097/ALN.0000000000005088. PMID: 38775960; PMCID: PMC11389889.
“EEG analysis enables visualization of the pharmacodynamic effects of γ-aminobutyric acid–mediated (GABAergic) anesthetic agents on the cerebral cortex. The complexity of interpreting EEG changes in anesthetized neonates is due to the need to consider both the particularities of the EEG trace in the neonate, whose cerebral cortex is still maturing, and the possible pharmacodynamic specificities of anesthetic agents on this immature brain. Conventional EEG, obtained by electrodes on the scalp, captures a low-amplitude electrical signal representing the summation of the electrical potentials of cortical neurons, organized in columns. In awake adults, there is rich, desynchronized spontaneous activity, typically resulting in a low-amplitude EEG with rapid oscillations, dominated by alpha and beta types. GABAergic anesthetic agents induce dose-dependent neuronal inhibition, electrically translated by synchronization of neuronal discharges leading to characteristic profiles on the cortical EEG. These profiles show a dose-dependent slowing and increase in the amplitude of the slow oscillations, up to the occurrence of burst suppression and possible isoelectric tracing. Functionally, the adult brain is characterized by rich and complex neuronal activity, organized into networks with thalamo-cortical and cortico-cortical connections, involved in consciousness and reactivity to ascending stimuli. EEG tracings recorded in anesthetized children greater than 1 yr old are close to those observed in adults, differing mainly in oscillation amplitude changes.3”
“Conversely, in neonates, cerebral immaturity is associated with a distinctive and evolving EEG pattern in the first months of life. The dynamic processes of cerebral maturation result in changes in cortical neuron activity and hence in the EEG patterns. Further, pharmacologically, γ-aminobutyric acid initially has an excitatory function, which changes to an inhibitory function during the third trimester of pregnancy.”2
In the neonate, cortical suppression occurs at very low doses of either an inhaled general anesthetic or propofol. “Whether this cortical inhibition reflects the depth of the hypnotic component of anesthesia depends on the definition of hypnosis and the brain structures involved—isolated cerebral cortex, which is unlikely, or cortex and subcortex, which is more likely. Subcortical activity in anesthetized neonates is probably less easily inhibited than cortical activity. Clinically, it is not uncommon to observe a hemodynamic or motor response to nociceptive stimuli in neonates, even with an isoelectric EEG. This suggests greater cortical sensitivity to GABAergic anesthetic effects compared to subcortical regions.”2 To block these subcortical responses to nociceptive stimuli, opioids like fentanyl or remifentanil may be an essential component of an anesthetic.
The systematic review article by Corlette et al. “aimed to summarize current literature reporting patterns of EEG during general anesthesia in term neonates aged 37 to 44 weeks postmenstrual age.”1 The bottom line is that there isn’t a lot of published data on this issue, and what is out there seems to suggest anesthetic drug dose and hypothermia are associated with the development of low voltage. As the authors also point out, whether the anesthesia-induced effects are cortical vs. subcortical or at the level of the spinal cord are also unclear, and may suggest interesting mechanistic differences in anesthetic action in neonates compared to older children and adults. The accompanying editorial suggests that anesthesia-induced hypotension leading to impaired cerebral perfusion may also be important contributor to low voltage patterns, and these data were not studied in the systematic review. If EEG monitoring is as important as we think it is, much further research work needs to be done and is an incredible opportunity for some you reading this.
Send your thoughts and comments to Myron who will post in a Friday reader response.
References
1. Corlette SJ, Walker SM, Cornelissen L, Brasher C, Bower J, Davidson AJ. Changes in the Term Neonatal Electroencephalogram with General Anesthesia: A Systematic Review with Narrative Synthesis. Anesthesiology 2024;141(4):670-680. (In eng). DOI: 10.1097/aln.0000000000005088.
2. Constant I. Challenge of Neonatal Anesthesia: Which Optimal EEG Target? Anesthesiology 2024;141(4):632-634. (In eng). DOI: 10.1097/aln.0000000000005157.
3. Rigouzzo A, Khoy-Ear L, Laude D, et al. EEG profiles during general anesthesia in children: A comparative study between sevoflurane and propofol. Paediatric anaesthesia 2019;29(3):250-257. (In eng). DOI: 10.1111/pan.13579.